家族性蛋白 S 缺乏症诱发的抗凝紊乱的蛋白质组和代谢组图谱分析
IF 3.8
2区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
蛋白 S 缺乏症(PSD)是一种常染色体显性遗传疾病,以先天性血栓性疾病为特征。目前对 PSD 的研究还很有限,因此还不清楚异常凝血过程中的分子变化。研究人员利用液相色谱法和气相色谱-质谱法对 PSD 患者的血浆进行了蛋白质组学和代谢组学分析。然后通过单变量统计分析筛选出 PSD 的差异蛋白质和代谢物,并使用 ingenuity pathway analysis (IPA) 平台进行网络分析。结果表明,PSD 的蛋白质组和代谢组受到了明显的干扰,其中凝血和补体级联的生物通路受到的影响最大。在 PSD 期间,抗凝血蛋白的总体水平下降,凝血酶生成的负调控作用减弱,导致纤维蛋白凝块的形成和血小板聚集。此外,9种差异蛋白与蛋白S显著相关,包括A2M、AGT、APOE、FGG、GPLD1、IGHV1-69、CFHR5、CPN2和CA1。生物网络表明,急性期反应、FXR/RXR激活、5-羟色胺受体信号转导和p70S6K信号转导等通路与PSD相关,表明炎症免疫和脂质代谢的相互作用紊乱。这些发现可能有助于了解家族性 PSD 的现有功能分子和生物通路,并有助于改善治疗。数据可通过 ProteomeXchange(标识符为 PXD055111)和 MetaboLights(参考号为 MTBLS2653)获取。本文章由计算机程序翻译,如有差异,请以英文原文为准。
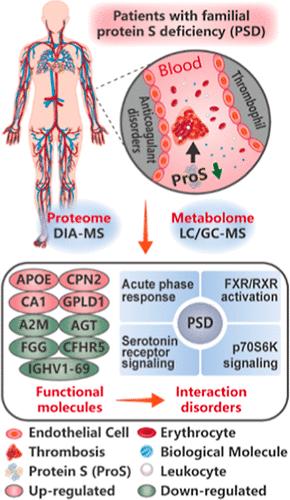
Proteome and Metabolome Profiling of Anticoagulant Disorders Induced by Familial Protein S Deficiency
Protein S deficiency (PSD) is an autosomal dominant disorder characterized by congenital thrombophilia. Studies on PSD are limited yet, resulting in a lack of clarity about molecular changes during abnormal coagulation. Proteomics and metabolomics analyses were conducted on the plasma of PSD patients based on liquid and gas chromatography–mass spectrometry (LC- and GC–MS). Differential proteins and metabolites of PSD were then filtered by univariate statistical analysis and subjected to network analysis using the ingenuity pathway analysis (IPA) platform. The proteome and metabolome of PSD were obviously disturbed, and the biological pathway of coagulation and complement cascades was the most affected. During PSD, overall levels of anticoagulant protein decreased and negative regulation of thrombin production was reduced, causing the formation of fibrin clots and platelet aggregation. Furthermore, 9 differential proteins correlated significantly with protein S, comprising A2M, AGT, APOE, FGG, GPLD1, IGHV1–69, CFHR5, CPN2, and CA1. The biological networks suggested that the pathways of acute phase response, FXR/RXR activation, serotonin receptor signaling, and p70S6K signaling were associated with PSD, indicating an interaction disorder of inflammatory immune and lipid metabolism. The findings may contribute to knowledge of available functional molecules and biological pathways of familial PSD and help with treatment improvement. Data are available via ProteomeXchange with identifier PXD055111 and MetaboLights with reference number MTBLS2653.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Proteome Research
生物-生化研究方法
CiteScore
9.00
自引率
4.50%
发文量
251
审稿时长
3 months
期刊介绍:
Journal of Proteome Research publishes content encompassing all aspects of global protein analysis and function, including the dynamic aspects of genomics, spatio-temporal proteomics, metabonomics and metabolomics, clinical and agricultural proteomics, as well as advances in methodology including bioinformatics. The theme and emphasis is on a multidisciplinary approach to the life sciences through the synergy between the different types of "omics".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


