通过双氧化自由基-极性交叉,用 H2O 对苄基和烯丙基 C-H 键进行无金属、无氧化剂的羰基化反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在温和条件下选择性地、可控地对无反应的 C(sp3)-H 键进行官能化,是有机合成和制药行业一个非常理想但又极具挑战性的目标。在此,我们报告了一种前所未有的以可见光为介导的、不含金属和氧化剂的苄基和烯丙基 C-H 键与 H2O 的羰基化反应。有机光催化剂 4CzIPN 和硫醇的协同组合,能以中等到极好的产率和良好的官能团兼容性,将不同阵列的苄基和烯丙基 C-H 键转化为羰基。此外,该方法还能将胺氧化成醛。从机理上讲,氧化自由基-极性交叉(ORPC)过程产生醇中间体,然后经过另一个 ORPC 过程生成羰基产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
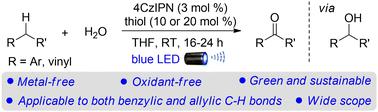
Metal- and oxidant-free carbonylation of benzylic and allylic C–H bonds with H2O via dual oxidative radical-polar crossover†
The selective and controllable functionalization of unreactive C(sp3)–H bonds under mild conditions is a highly desirable yet challenging goal in both organic synthesis and pharmaceutical industry. Herein, we report an unprecedented visible-light mediated metal- and oxidant-free carbonylation of both benzylic and allylic C–H bonds with H2O. The synergistic combination of an organophotocatalyst 4CzIPN and a thiol enables the transformation of diverse arrays of benzylic and allylic C–H bonds into carbonyls in moderate to excellent yields with good functional group compatibility. Moreover, the oxidation of amines to aldehydes has also been realized by this protocol. Mechanistically, an oxidative radical-polar crossover (ORPC) process affords an alcohol intermediate, which then undergoes another ORPC process to furnish the carbonyl product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


