树枝状 ZSM-5 沸石作为单萜环氧化物价值化的高活性催化剂
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究了树枝状 ZSM-5 沸石在单萜烯环氧化物(包括柠檬烯-1,2-环氧化物 (LE)、α-蒎烯环氧化物和β-蒎烯环氧化物)异构化过程中的应用,这些单萜烯环氧化物可产生用于香料、化妆品和药品的高价值化合物。使用 XRD、Ar 物理吸附、吡啶-傅立叶变换红外光谱、TEM、傅立叶变换红外光谱/DTBPyr 和 27Al MAS NMR 对新鲜催化剂进行了全面表征。与传统和分层 ZSM-5 材料相比,结晶时间为 4 天的树枝状沸石(d-ZSM-5/4d)是最活跃的材料,其 LE 异构化的周转频率值为 4.4 分钟-1。同样,这种中孔/外表面积最大(360 m2 g-1)、中孔尺寸分布最窄的材料也获得了二氢香芹酮(DHC,63%,70 °C,2 小时)、樟脑烯醛(72.4%,70 °C,5 分钟)和香叶醛(47.7%,50 °C,5 分钟)的高产率。TOF 值与外部布氏酸位点浓度之间存在直接关系,这表明存在很强的立体/扩散限制,而树枝状沸石则大大克服了这一限制。与顺式烯丙基醚相比,反式烯丙基醚的反应活性较低,这是因为氧原子的立体阻碍较大。对溶剂影响的研究表明,非极性溶剂有利于烯烃的反应速率,而中等极性溶剂则会高度选择性地形成 DHC。d-ZSM-5/4d 样品的催化活性可以通过空气煅烧完全恢复,因此被证明是坚固的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
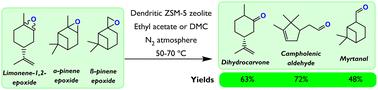
Dendritic ZSM-5 zeolites as highly active catalysts for the valorization of monoterpene epoxides
Dendritic ZSM-5 zeolites were investigated in the isomerization of monoterpene epoxides, including limonene-1,2-epoxide (LE), α-pinene epoxide, and β-pinene epoxide, which yields high-value compounds used in fragrances, cosmetics, and pharmaceuticals. The fresh catalysts were thoroughly characterized using XRD, Ar physisorption, pyridine-FTIR, TEM, FTIR/DTBPyr, and 27Al MAS NMR. In comparison with conventional and hierarchical ZSM-5 materials, the dendritic zeolite with a crystallization time of 4 days (d-ZSM-5/4d) was the most active material, with a turnover frequency value of 4.4 min−1 for LE isomerization. Likewise, remarkable yields of dihydrocarvone (DHC, 63%, 70 °C, 2 h), campholenic aldehyde (72.4%, 70 °C, 5 min), and myrtanal (47.7%, 50 °C, 5 min) were obtained with this material that exhibited the largest mesopore/external surface area (360 m2 g−1), showing also the narrowest mesopore size distribution. A direct relationship was observed between the TOF values and the concentration of external Brønsted acid sites, showing the presence of strong steric/diffusional limitations that are greatly overcome with the dendritic zeolites. The lower reactivity of trans-LE compared to cis-LE was attributed to the larger steric hindrance of the oxygen atom. Exploration of the solvent influence revealed that the reaction rate of LE was favored by non-polar solvents, while highly selective DHC formation occurred in the solvents of medium polarity. The d-ZSM-5/4d sample was shown to be robust because catalytic activity could be completely recovered by air calcination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


