以 SF6 为氟化试剂,利用可见光诱导光催化苯甲醇的脱氧氟化反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氟原子能显著增强有机分子的代谢稳定性和生物利用度,因此氟化苄基是药物或生物活性分子中不可或缺的骨架。在此,我们采用六氟化硫(SF6)作为高效氟化试剂,在可见光 LED 光照射下,利用低剂量光催化剂 4CzIPN 实现了广泛存在的苄醇的亲核氟化反应。该反应与多种底物骨架兼容,对空气和湿气不敏感,实现了有效温室气体资源 SF6 的降解和利用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
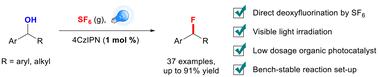
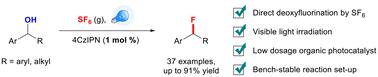
Visible light-induced photocatalytic deoxyfluorination of benzyl alcohol using SF6 as a fluorinating reagent†
As fluorine atoms significantly strengthen the metabolic stability and bioavailability of organic molecules, benzyl fluoride is found as an essential skeleton in pharmaceuticals or biologically active molecules. Here, we employ sulfur hexafluoride (SF6) as an efficient fluorinating reagent, achieving nucleophilic fluorination of widely available benzyl alcohols under visible LED light irradiation with a low dosage of photocatalyst 4CzIPN. The reaction is compatible with several substrate backbones and is not air- or moisture-sensitive, realizing the degradation and utilization of SF6, a potent greenhouse gas resource.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


