基于 BODIPYS 的荧光标记监测 TNBC 的自噬溶酶体和脂滴
IF 3.5
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
溶酶体酶和脂滴的大量积累与乳腺癌有关。BODIPYs 可监测溶酶体和脂滴,在癌细胞中起到自噬激活剂的作用。我们合成了 BD-1 和 BD-2,并通过质谱、紫外可见光光谱、荧光光谱和核磁共振光谱对其进行了表征。在 BODIPYs 中,咔唑基团的作用体现在较大的斯托克斯位移(2143-1651 cm-1)和红色荧光上。在白光下,BODIPYs 能产生 ROS 并诱导三阴性乳腺癌细胞(MDA-MB-231)自噬。共聚焦实验显示,BD-1 和 BD-2 优先共定位在溶酶体和脂滴中。自噬溶酶体和脂滴在细胞质中释放出 Ca2+ 离子,这在 Fura-2M 染料的蓝色荧光中很明显。BD-1 与自噬抑制剂结合使用,在白光下对三阴性乳腺癌细胞具有出色的光毒性(5.57 μM)。这项工作证明了 BODIPYs 作为治疗剂用于 TNBC 光动力疗法的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
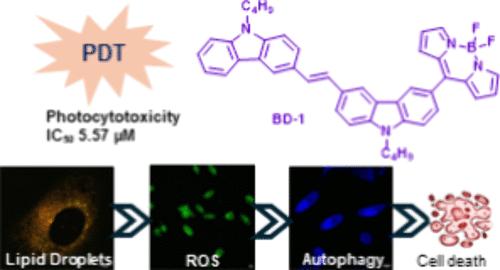
BODIPYS Based Fluorescent Markers To Monitor Autophagic Lysosomes and Lipid Droplets in TNBC
Lysosomal enzymes and high accumulation of lipid droplets are associated with breast cancer. The lysosomes and lipid droplets were monitored by BODIPYs, acting as autophagy activators in cancer cells. BD-1 and BD-2 were synthesized and characterized by Mass, UV–visible, fluorescence, and NMR spectroscopies. In BODIPYs, the effect of carbazole groups was reflected by the large Stokes shifts (2143–1651 cm–1) and red fluorescence. BODIPYs generated ROS and induced autophagy in triple negative breast cancer cells (MDA-MB-231) under white light. Confocal experiments revealed that BD-1 and BD-2 preferentially colocalized in lysosomes and lipid droplets. Autophagic lysosomes and lipid droplets released Ca2+ ions in the cytoplasm, which was evident with blue fluorescence of Fura-2M dye. In combination with an autophagy inhibitor, BD-1 displayed excellent photocytotoxicity (5.57 μM) on triple negative breast cancer cells under white light. This work demonstrates the potential of BODIPYs as theranostic agents for the photodynamic therapy against TNBC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


