5-N-Arylaminocarbonyl-6-Aryl-4-Methyl-1,2,3,6-Tetrahydropyrimidine-2-Thiones 的空间结构和抗炎活性
IF 1
4区 医学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
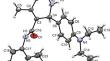
研究了 17 种 5-N-芳基氨基羰基-6-芳基-4-甲基-1,2,3,6-四氢嘧啶-2-硫酮衍生物的抗炎活性。其中一种化合物的分子结构已经确定。确定了 10 种化合物,它们对实验动物炎症反应的抑制率在 56% 至 81% 之间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatial Structure and Anti-Inflammatory Activity of 5-N-Arylaminocarbonyl-6-Aryl-4-Methyl-1,2,3,6-Tetrahydropyrimidine-2-Thiones
The anti-inflammatory activity of 17 derivatives of 5-N-arylaminocarbonyl-6-aryl-4-methyl-1,2,3,6-tetrahydropyrimidine-2-thiones was studied. The molecular structure of one of them was established. Ten compounds that inhibited the inflammatory reaction in experimental animals in the range 56-81% were identified.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmaceutical Chemistry Journal
CHEMISTRY, MEDICINAL-PHARMACOLOGY & PHARMACY
CiteScore
1.30
自引率
22.20%
发文量
226
审稿时长
3-8 weeks
期刊介绍:
Pharmaceutical Chemistry Journal is a monthly publication devoted to scientific and technical research on the creation of new drugs and the improvement of manufacturing technology of drugs and intermediates. International contributors cover the entire spectrum of new drug research, including:
methods of synthesis;
results of pharmacological, toxicological, and biochemical studies;
investigation of structure - activity relationships in prediction of new compounds;
methods and technical facilities used; and
problems associated with the development of ecologically safe and economically feasible methods of industrial production.
In addition, analytical reviews of the international literature in the field provide coverage of the most recent developments around the world.
Pharmaceutical Chemistry Journal is a translation of the Russian journal Khimiko-Farmatsevticheskii Zhurnal. The Russian Volume Year is published in English from April.
All articles are peer-reviewed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


