4-(3-酰基-2-芳基-4-羟基-5-氧代-2,5-二氢-1H-吡咯-1-基)苯甲酸的合成与镇痛活性
IF 1
4区 医学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
4- (3-酰基-2-芳基-4-羟基-5-氧代-2,5-二氢-1H-吡咯-1-基)苯甲酸是由酰基丙酮酸甲酯、芳香醛和 4-氨基苯甲酸 (PABA) 三组分反应合成的。利用红外光谱、PMR 光谱和 13C NMR 光谱确定了这些化合物的结构。合成的化合物具有明显的镇痛活性。杂环 3 位上的酰基取代基提高了镇痛活性。所有研究的化合物实际上都是无毒的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
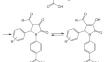
Synthesis and Analgesic Activity of 4-(3-Acyl-2-Aryl-4-Hydroxy-5-Oxo-2,5-Dihydro-1H-Pyrrol-1-yl)Benzoic Acids
4-(3-Acyl-2-aryl-4-hydroxy-5-oxo-2,5-dihydro-1H-pyrrol-1-yl)benzoic acids were synthesized by three- component reactions of acylpyruvic acid methyl esters, aromatic aldehydes, and 4-aminobenzoic acid (PABA). The structures of the compounds were determined using IR, PMR, and 13C NMR spectroscopy. Pronounced analgesic activity of the synthesized compounds was revealed. An aroyl substituent in the 3-position of the heterocycle was shown to increase the analgesic activity. All studied compounds were practically nontoxic.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmaceutical Chemistry Journal
CHEMISTRY, MEDICINAL-PHARMACOLOGY & PHARMACY
CiteScore
1.30
自引率
22.20%
发文量
226
审稿时长
3-8 weeks
期刊介绍:
Pharmaceutical Chemistry Journal is a monthly publication devoted to scientific and technical research on the creation of new drugs and the improvement of manufacturing technology of drugs and intermediates. International contributors cover the entire spectrum of new drug research, including:
methods of synthesis;
results of pharmacological, toxicological, and biochemical studies;
investigation of structure - activity relationships in prediction of new compounds;
methods and technical facilities used; and
problems associated with the development of ecologically safe and economically feasible methods of industrial production.
In addition, analytical reviews of the international literature in the field provide coverage of the most recent developments around the world.
Pharmaceutical Chemistry Journal is a translation of the Russian journal Khimiko-Farmatsevticheskii Zhurnal. The Russian Volume Year is published in English from April.
All articles are peer-reviewed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


