生物炭对 Fe(II)aq 催化的铁酸盐转化的影响
IF 2.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
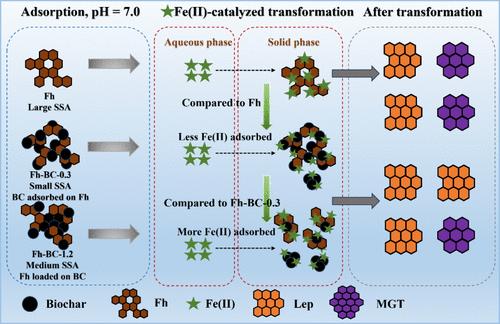
非晶态铁(Fe)氢氧化物水合物通过铁(II)非生物转化为结晶性铁(III)矿物,在全球铁循环中发挥着重要作用。在自然环境中,Fe(III) 矿物通常与有机物共存,有机物会改变其矿化途径和副产品。然而,生物炭等外源有机物对铁(III)矿物转化的影响仍不清楚。本研究合成了一系列具有不同 C/Fe 摩尔比的铁水物-生物炭复合物(Fh-BCs),以评估 Fh-BCs 在中性厌氧条件下的非生物铁(II)催化矿化转化。在合成 Fh-BC 的过程中,生物炭通过吸附在 C/Fe 比为 0.3 的 Fh 上与 Fh 形成复合物,而 Fh 负载在 C/Fe 比为 1.2 的生物炭上则生成 Fh-BC-1.2 复合物。与纯 Fh 相比,所有 Fh-BC 的比表面积和总孔隙体积都有所下降。Fh 转化过程中次生矿物的形成取决于 C/Fe 比率。在 Fh-BC + Fe(II)处理中,生物炭抑制了磁铁矿(Mgt)的形成,但没有抑制鳞片闪石(Lep)的形成。然而,磁铁矿形成的抑制水平与 C/Fe 比率呈负相关。根据相变动力学分析随时间变化的铁(II)和易溶铁(III)浓度(易溶铁(III))的实验表明,生物炭占据了 Fh 表面的吸附位点,抑制了 Fh 与铁(II)之间的电子交换,从而阻止了 Fh 的水解-重沉淀转化为更稳定的矿物相。高 C/Fe 比适度增强了 Fh 的转化,这归因于 Fh 负载结构促进了铁(II)的吸附,并促进了铁(II)和 Fh 之间有效的电子传递。这些结果表明,生物炭改性的 Fh 有利于稳定的结晶铁(III)矿物的相变,可能为了解富碳土壤环境中铁/碳循环的地球化学行为提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of Biochar on the Fe(II)aq-Catalyzed Transformation of Ferrihydrite
The abiotic transformation of an amorphous iron (Fe) hydroxide hydrate to more crystalline Fe(III) minerals by Fe(II) plays an essential role in global Fe cycling. In natural environments, Fe(III) minerals generally coexist with organic matter, which modulates their mineralization pathways and byproducts. Nevertheless, the effect of exogenous organic matter, such as biochar, on the transformation of Fe(III) minerals remains unclear. In this study, a series of ferrihydrite-biochar complexes (Fh-BCs) with various C/Fe molar ratios were synthesized to evaluate the abiotic Fe(II)-catalyzed mineralogical transformation of Fh-BCs under neutral anaerobic conditions. During the synthesis of Fh-BC, biochar formed a complex with Fh through adsorption onto Fh at a C/Fe ratio of 0.3, whereas Fh loaded onto biochar with a C/Fe ratio of 1.2 generated the Fh-BC-1.2 complex. Compared to pure Fh, the specific surface area and total pore volume decreased in all of the Fh-BCs. The secondary mineral formation during Fh transformation depended on the C/Fe ratios. Biochar inhibited the formation of magnetite (Mgt) but not lepidocrocite (Lep) in the treatments of Fh-BC + Fe(II). However, the inhibition level of Mgt formation was negatively correlated with the C/Fe ratio. Experiments analyzing the time-dependent concentrations of Fe(II) and labile Fe(III) (Fe(III)labile) against the kinetics of phase transformation showed that the occupation of adsorption sites on the surface of Fh by biochar inhibited electron exchange between Fh and Fe(II), thereby preventing the hydrolysis–reprecipitation of Fh into the more stable mineral phase. High C/Fe ratios modestly enhanced the transformation of Fh, which was attributed to the Fh-loaded structure that facilitated Fe(II) sorption and promoted efficient electron transfer between Fe(II) and Fh. These results indicate that biochar-modified Fh is favorable to the phase transformation of stable crystalline Fe(III) minerals, possibly providing new insight into the geochemical behavior of Fe/C cycling in carbon-rich soil environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Earth and Space Chemistry
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
5.30
自引率
11.80%
发文量
249
期刊介绍:
The scope of ACS Earth and Space Chemistry includes the application of analytical, experimental and theoretical chemistry to investigate research questions relevant to the Earth and Space. The journal encompasses the highly interdisciplinary nature of research in this area, while emphasizing chemistry and chemical research tools as the unifying theme. The journal publishes broadly in the domains of high- and low-temperature geochemistry, atmospheric chemistry, marine chemistry, planetary chemistry, astrochemistry, and analytical geochemistry. ACS Earth and Space Chemistry publishes Articles, Letters, Reviews, and Features to provide flexible formats to readily communicate all aspects of research in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


