一步甲醇法二氧化碳转化为汽油:全面回顾与重要展望
IF 5.3
3区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
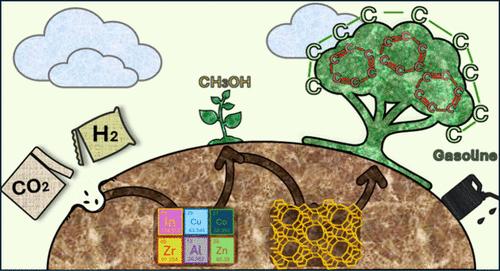
减少对化石原料的需求是支持我们社会所需的能源和环境转型的最佳途径之一。利用二氧化碳,更具体地说,将二氧化碳转化为碳氢化合物,是减少二氧化碳排放和获得传统化石燃料生产的碳中性燃料和化学品的一条有吸引力的途径。实现这一目标的方法之一是将二氧化碳转化为甲醇,然后再将甲醇转化为碳氢化合物。迄今为止,这些过程主要是作为单独的步骤来研究的,一种观点是按顺序进行操作。不过,串联催化也可以一步完成。这种催化剂通常是用于第一个反应的氧化物和催化第二个反应的酸性沸石。针对这两个独立步骤已经研究了许多催化剂,但在以汽油范围为目标的串联催化中,只有少数催化剂得到了研究。在氧化物中,ZnZrOx 和 In2O3 占主导地位,而金属 InCo 也有其优点。当这些氧化物与沸石(通常为 ZSM-5)结合使用时,会产生有趣的选择性和产率。我们尚未清楚地了解这些系统背后的机理;不过,我们将对已取得的机理结果进行总结,并为进一步的研究提供见解。虽然已经对床层配置或数量接近度等参数进行了研究,但还需要进行更多的研究,尤其是在研究复杂的动力学时。这篇 "二氧化碳直接转化为汽油范围内的碳氢化合物"(包括芳烃)的综述旨在将各点联系起来,同时强调仍需深入了解的方面,并指出实用的见解和观点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
One Step Methanol-Mediated CO2 Conversion to Gasoline: Comprehensive Review and Critical Outlook
Decreasing our demand for fossil feedstock is one of the best ways to support the energy and environmental transitions that are needed for our society. CO2 utilization and, more specifically, CO2 conversion to hydrocarbons are an attractive route to reduce CO2 emissions and to obtain carbon-neutral fuels and chemicals that are conventionally produced from fossil fuels. One way to achieve that is through the conversion of CO2 to methanol, followed by methanol conversion to hydrocarbons. So far, these processes have mainly been studied as separate steps, and one view is to sequentially operate them. However, it is possible to perform it in one step, in tandem catalysis. Such catalysts are usually an oxide for the first reaction combined with an acidic zeolite that catalyzes the second reaction. Many catalysts have been researched for the two separate steps but only a few have been studied for the tandem when the gasoline range is the target. Among the oxides, ZnZrOx and In2O3 dominate the art, while more metallic InCo also has its merits. These lead to interesting selectivities and yields when combined with a zeolite (usually ZSM-5). A clear understanding of the mechanism behind these systems has not been reached; yet, we deliver a summary of the achieved mechanistic results and offer insights for further studies. While parameters such as bed configuration or amount proximity have been studied, more research is needed, especially when looking at the complex kinetics. This “direct CO2 to gasoline range hydrocarbons” (including aromatics) review aims to connect dots while highlighting the aspects that still need a deeper understanding, and it also pinpoints practical insights and perspectives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy & Fuels
工程技术-工程:化工
CiteScore
9.20
自引率
13.20%
发文量
1101
审稿时长
2.1 months
期刊介绍:
Energy & Fuels publishes reports of research in the technical area defined by the intersection of the disciplines of chemistry and chemical engineering and the application domain of non-nuclear energy and fuels. This includes research directed at the formation of, exploration for, and production of fossil fuels and biomass; the properties and structure or molecular composition of both raw fuels and refined products; the chemistry involved in the processing and utilization of fuels; fuel cells and their applications; and the analytical and instrumental techniques used in investigations of the foregoing areas.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


