作为乙烯聚合前催化剂的对芳基取代的氨基吡啶铬络合物:在缺乏结构数据的情况下通过实验和密度函数研究考察物理氧化态问题
IF 2.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
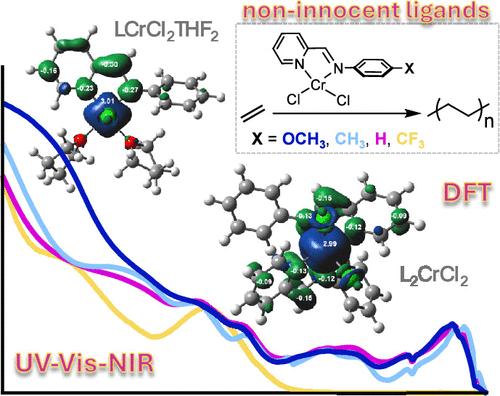
对亚胺吡啶铬配合物的研究揭示了它们作为(二)烯烃聚合前催化剂的潜力(Organometallics 2018, 37, 4827-4840)。事实证明,配体(L)处于单阴离子自由基状态,而 Cr 处于三价物理氧化态,Cr 与配体之间的电子转移是促进乙烯聚合的基本要素,而配体与未取代的醛亚胺(Cr-pH,其中 X = H)之间的电子转移是促进乙烯聚合的基本要素。在缺乏结构数据的情况下,我们通过紫外-可见-近红外光谱(UV-vis-NIR)和密度泛函理论进行了详细研究,以阐明驱动 Cr-pH 中电子转移的结构和电子特征。在单配位形式的金属配位层中存在两个四氢呋喃或形成双配位物种时,会促进 Cr 到 L 的电子转移。Cr-pH 的紫外-可见-近红外光谱与电喷雾电离质谱数据相结合,表明存在多种物种。随后,我们在 p-N 芳基 4 位上添加了不同的取代基(即 CH3、OCH3 和 CF3),从而扩大了铬配合物库。Cr-pCH3 和 Cr-pOCH3 显示出与 Cr-pH 类似的电子特征,而 Cr-pCF3 则是一个例外,可能只包含单锂化种类。Cr-pCF3 是最活跃的催化剂,可在 40 °C 时生成分子量分布单一且狭窄的超高分子量聚(乙烯)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
p-Aryl-Substituted Iminopyridine Chromium Complexes as Precatalysts for Ethylene Polymerization: The Question of the Physical Oxidation State Examined by Experimental and Density Functional Study in the Absence of Structural Data
Investigations into iminopyridine chromium complexes have unveiled their potential as precatalysts for the polymerization of (di)olefins (Organometallics 2018, 37, 4827–4840). A Cr-to-ligand electron transfer, with the ligand (L) in the monoanionic radical state and Cr in a physical trivalent oxidation state, proved to be fundamental for facilitating ethylene polymerization by the complex with the unsubstituted aldimine (Cr-pH, where X = H). In the absence of structural data, we embarked on a detailed study by ultraviolet–visible–near-infrared (UV–vis–NIR) spectroscopy and density functional theory to elucidate the structural and electronic features driving electron transfer in Cr-pH. The Cr-to-L electron transfer is facilitated by the presence of two tetrahydrofurans in the metal’s coordination sphere in the monoligated form or by the formation of a bis-ligated species. The UV–vis–NIR spectra of Cr-pH, coupled with electrospray ionization mass spectroscopy data, indicate the coexistence of multiple species. Successively, we enlarged the library of chromium complexes by appending different substituents (i.e., CH3, OCH3, and CF3) at the p-N-aryl 4-position. While Cr-pCH3 and Cr-pOCH3 display electronic features analogous to those of Cr-pH, Cr-pCF3 stands out as an exception, likely containing only monoligated species. Cr-pCF3 emerged as the most active catalyst, producing ultra-high-molecular weight poly(ethylene) with a unimodal and narrow molecular weight distribution event at 40 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organometallics
化学-无机化学与核化学
CiteScore
5.60
自引率
7.10%
发文量
382
审稿时长
1.7 months
期刊介绍:
Organometallics is the flagship journal of organometallic chemistry and records progress in one of the most active fields of science, bridging organic and inorganic chemistry. The journal publishes Articles, Communications, Reviews, and Tutorials (instructional overviews) that depict research on the synthesis, structure, bonding, chemical reactivity, and reaction mechanisms for a variety of applications, including catalyst design and catalytic processes; main-group, transition-metal, and lanthanide and actinide metal chemistry; synthetic aspects of polymer science and materials science; and bioorganometallic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


