钼酸阴离子对 d-葡萄糖酮的作用:通过 C1-C2 转位催化转化为醛酸盐
IF 3.7
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
用钼酸钠在 90° 条件下处理烯酮(醛糖-2-酮)、d-[1-13C]葡糖酮 (11) 30 小时后,生成了比例为 85:15 的 d-[2-13C]葡糖酸盐 (22) 和 d-[2-13C]甘露酸盐 (32) 混合物,这表明反应是通过 C1-C2 转位进行的,与之前观察到的醛糖反应类似。与 1 的几种单 13C 标记和双 13C 标记同位素的反应证实了这种转位。与醛糖反应不同的是,与 1 的反应是不可逆的,这可能是由于带负电荷的催化活性双钼酸盐与带负电荷的醛酸盐产物之间存在静电排斥。2 和 3 的生成是通过形成两种结构不同的双钼酸盐复合物来介导的,一种生成葡萄糖异构体,另一种生成甘露异构体。当 11 被用作反应物时,还观察到了反应副产物,特别是 d-[1-13C]阿拉伯糖(41)和[13C]甲酸盐(5)。这些副产物似乎是由双钼酸盐复合物通过与形成醛酸盐的途径竞争的其他途径分解形成的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
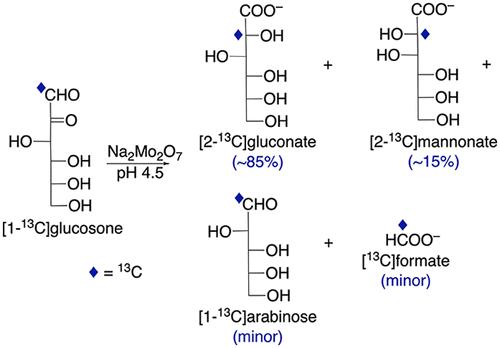
Action of Molybdate Anion on d-Glucosone: Catalytic Conversion to Aldonates Involving C1–C2 Transposition
Treatment of the osone (aldos-2-ulose), d-[1-13C]glucosone (11), with sodium molybdate at 90° for 30 h gave a mixture of d-[2-13C]gluconate (22) and d-[2-13C]mannonate (32) in an 85:15 ratio, indicating that the reaction proceeds with C1–C2 transposition similar to that observed previously with aldoses. Reactions with several singly and doubly 13C-labeled isotopomers of 1 confirmed this transposition. In contrast to the aldose reaction, the reaction with 1 is irreversible, presumably due to electrostatic repulsion between the negatively charged catalytically active bimolybdate species and the negatively charged aldonate product. The production of 2 and 3 is mediated by the formation of two structurally distinct bimolybdate complexes, one producing the gluco isomer and the other producing the manno isomer. Reaction byproducts were also observed, specifically d-[1-13C]arabinose (41) and [13C]formate (5), when 11 was used as the reactant. These byproducts appear to form from the breakdown of bimolybdate complexes via alternative pathways that compete with those responsible for aldonate formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Omega
Chemical Engineering-General Chemical Engineering
CiteScore
6.60
自引率
4.90%
发文量
3945
审稿时长
2.4 months
期刊介绍:
ACS Omega is an open-access global publication for scientific articles that describe new findings in chemistry and interfacing areas of science, without any perceived evaluation of immediate impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


