AILDE 计算机辅助发现以环氧化酶-2 为靶点的新型布洛芬香豆素抗肿瘤先导化合物
IF 3.7
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从三个布洛芬-香豆素化合物开始,我们利用计算自动硅学配体定向进化(AILDE)方法,通过引入不同的取代基,设计了18个针对环氧化酶-2(COX-2)的衍生化合物。经过合成和活性测试,我们发现 6 个代表性化合物对 COX-2 具有微摩尔酶抑制活性。此外,还有 16 个化合物对宫颈癌细胞具有一定的抑制活性。在这些化合物中,6c(IC50 = 0.606 μM,HeLa)和 7g(IC50 = 0.783 μM,HeLa)表现出了极佳的活性,约为商业药物吉非替尼的 10 倍。根据分子模拟结果,香豆素环上的 6c 和 7g 的卤素原子能与 COX-2 形成卤素键,与它们的热门化合物 6a 和 7a 相比,活性显著提高。然而,这两种化合物在与 COX-1 结合时失去了关键的相互作用。计算结果显示,这两个化合物是选择性 COX-2 抑制剂,潜在选择性指数分别为 6 倍和 5 倍。化合物 6c 和 7g 对 HEK293 细胞的细胞活性表明,我们的化合物对正常细胞具有可接受的安全性。研究结果表明,6c 和 7g 可作为潜在的先导化合物用于进一步的荧光分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
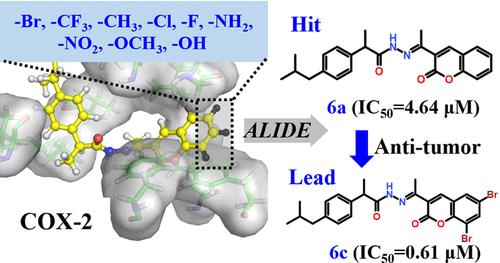
AILDE Computer-Aided Discovery of Novel Ibuprofen–Coumarin Antitumor Lead Compounds Targeting Cyclooxygenase-2
Starting from three ibuprofen–coumarin hit compounds, we designed 18 derivative compounds targeting cyclooxygenase-2 (COX-2) by introducing different substituents onto them by using the computational auto in silico ligand directing evolution (AILDE) method. After synthesizing and testing the activity, we found that 6 representative compounds have micromolar enzyme inhibitory activity against COX-2. Additionally, 16 compounds have shown certain inhibitory activity in cervical cancer cells. Among these compounds, 6c (IC50 = 0.606 μM, HeLa) and 7g (IC50 = 0.783 μM, HeLa) have exhibited excellent activity, which is approximately 10 times better than the commercial drug gefitinib. According to molecular simulation results, the halogen atoms of 6c and 7g on the coumarin ring can form halogen bonds with COX-2, which significantly improves their activity compared to their hit compounds 6a and 7a. However, the key interactions were lost in binding with COX-1. The calculation results revealed that the two compounds are selective COX-2 inhibitors, with potential selectivity indexes of 6-fold and 5-fold, respectively. The cell-based activity of compounds 6c and 7g toward HEK293 cells demonstrates that our compounds possess an acceptable safety toward normal cells. The results indicate that 6c and 7g can serve as potential lead compounds for further lucubrate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Omega
Chemical Engineering-General Chemical Engineering
CiteScore
6.60
自引率
4.90%
发文量
3945
审稿时长
2.4 months
期刊介绍:
ACS Omega is an open-access global publication for scientific articles that describe new findings in chemistry and interfacing areas of science, without any perceived evaluation of immediate impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


