全面探索溴酚衍生物:有望抗击 SA 和 MRSA 的抗菌剂
IF 3.7
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
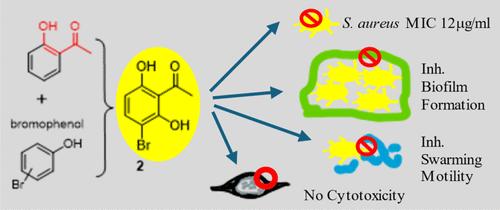
多年来,耐多药病原体导致的治疗失败率不断上升,其比率远远高于新抗生素药物的发现率。因此,本研究通过整合化学、微生物学和药理学,探讨了作为金黄色葡萄球菌和 MRSA 潜在抗菌剂的溴苯酚衍生物,以解决基于其官能团的溴苯酚抗菌活性方面的重大知识空白。令人惊讶的是,3-溴-2,6-二羟基苯乙酮(2)这一简单分子具有良好的抗金黄色葡萄球菌活性,甚至对耐药菌株 MRSA 也有很好的抑制作用。此外,化合物 2 还能抑制病原体常见的抗药性途径,即金黄色葡萄球菌和 MRSA 的生物膜形成。此外,化合物 2 的治疗指数高达 598,可视为对人类 HEK-293 细胞具有高选择性和低毒性。虽然这些化合物对革兰氏阴性菌铜绿假单胞菌的效果较差,但它们仍然对铜绿假单胞菌的毒力特性(如生物膜形成、脓青素产生和蜂拥运动)产生了一些影响。最后还讨论了结构-活性关系的硅学分析以及 ADMET 特性。该研究为溴酚类化合物的抗菌活性提供了一些启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Comprehensive Exploration of Bromophenol Derivatives: Promising Antibacterial Agents against SA and MRSA
The incidence of treatment failure due to multidrug-resistant pathogens elevated over the years; the rate is much higher than new antibiotic drug discovery. Therefore, bromophenol derivatives as potential antibacterial agents on Staphylococcus aureus and MRSA were explored in this research via integrating chemistry, microbiology, and pharmacology to address significant knowledge gaps pertaining to the antibacterial activity of bromophenols based on their functional groups. Surprisingly, a simple molecule, 3-bromo-2,6-dihydroxyacetophenone (2), exhibited good anti-S. aureus activity and even MRSA, a drug-resistant strain. In addition, compound 2 also inhibited a common resistant pathway of pathogens, biofilm formation of S. aureus and MRSA. Moreover, the therapeutic index of 2 is up to 598, which can be viewed as highly selective and having low toxicity to human HEK-293 cells. Although these compounds displayed less effectiveness for the Gram-negative bacterium, Pseudomonas aeruginosa, they still manifested some effects on the virulence properties of P. aeruginosa, such as biofilm formation, pyocyanin production, and swarming motility. In silico analyses of the structure–activity relationship as well as ADMET properties were discussed in the end. This study shed some light on the antibacterial activities of bromophenols.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Omega
Chemical Engineering-General Chemical Engineering
CiteScore
6.60
自引率
4.90%
发文量
3945
审稿时长
2.4 months
期刊介绍:
ACS Omega is an open-access global publication for scientific articles that describe new findings in chemistry and interfacing areas of science, without any perceived evaluation of immediate impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


