利用 LC-MS/MS 比较小鼠腹腔注射与口服六氢姜黄素的药代动力学和组织分布
IF 3.7
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
采用负离子模式电喷雾离子源,建立并验证了液相色谱-串联质谱(LC-MS/MS)测定小鼠血浆、大脑、肝脏和肾脏中六氢姜黄素(HHC)含量的方法。该方法的定量下限(LLOQ)为5纳克/毫升,在所有测试基质中的浓度范围为5-500纳克/毫升,线性关系良好。精密度评估报告显示,日内和日间测量的变异系数(CV%)均小于 13.19%,而所有质量控制水平的准确度范围为 95.13% 至 105.07%。HHC 提取回收率始终保持在 70.18% 到 93.28% 之间,CV% 偏差小于 15%。在对小鼠进行腹腔注射(IP)或口服单次给药后的 HHC 药代动力学评估时,采用了非室分析法。IP 给药(40 毫克/千克)后,Cmax 值是口服给药的 47.90 倍。IP 给药后约 5 分钟和口服给药后 15 分钟,血浆浓度达到峰值。IP 和口服给药后观察到的半衰期分别约为 1.52 小时和 2.17 小时。与 IP 给药途径相比,口服给药的相对生物利用度仅为 12.28%。此外,IP 给药后,大脑、肝脏和肾脏中的半衰期值差异不大,但高于血浆中的半衰期值。肝脏和肾脏中的HHC浓度最高,而大脑中的浓度最低,这表明HHC的疏水性阻碍了其通过血脑屏障。这项研究首次详细揭示了小鼠口服和 IP 给药后 HHC 的药代动力学和组织分布特征,为进一步关注 HHC 这一潜在候选新药奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
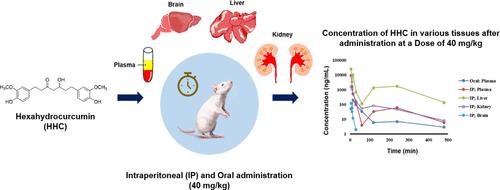
Comparative Pharmacokinetics and Tissue Distribution of Hexahydrocurcumin Following Intraperitoneal vs Oral Administration in Mice Using LC-MS/MS
A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed and validated to determine hexahydrocurcumin (HHC) levels in mouse plasma, brain, liver, and kidneys using a negative ion mode electrospray ionization (ESI) source. Demonstrating a lower limit of quantification (LLOQ) of 5 ng/mL, the method showed excellent linearity across a concentration range of 5–500 ng/mL in all tested matrices. Precision evaluations reported a coefficient of variation (CV%) of less than 13.19% for both intraday and interday measurements, while accuracy ranged from 95.13 to 105.07% across all quality control levels. HHC extraction recovery was consistently observed between 70.18 and 93.28%, with a CV% deviation of less than 15%. In the pharmacokinetic evaluation of HHC in mice following a single intraperitoneal (IP) or oral administration, a noncompartment analysis was utilized. After IP administration (40 mg/kg), the Cmax value was 47.90 times higher than that achieved via oral administration. Peak plasma concentrations were observed approximately 5 min post-IP and 15 min post-oral dosing. The observed half-lives after these administrations were approximately 1.52 and 2.17 h for IP and oral routes, respectively. Oral administration revealed a relative bioavailability of only 12.28% compared with the IP route. Furthermore, following IP administration, the half-life values in brain, liver, and kidney were not significantly different but more than the half-life value found in plasma. The liver and kidney exhibited the highest concentrations of HHC, while the brain showed the least, suggesting that the hydrophobic nature of HHC impedes its passage through the blood–brain barrier. This study is the first to provide detailed insights into the pharmacokinetics and tissue distribution characteristics of HHC following oral and IP administration in mice, setting the stage for further focus on HHC as a potential new drug candidate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Omega
Chemical Engineering-General Chemical Engineering
CiteScore
6.60
自引率
4.90%
发文量
3945
审稿时长
2.4 months
期刊介绍:
ACS Omega is an open-access global publication for scientific articles that describe new findings in chemistry and interfacing areas of science, without any perceived evaluation of immediate impact.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


