钛酸铥的合成与热力学性质
IF 1.5
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
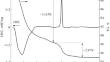
摘要 利用 DSC/TG、XRD 和电子显微镜研究了通过反向沉淀获得的氢氧化物前驱体在加热过程中热绿球石结构类型的钛酸铥结晶过程的温度阶段。在 2-1870 K 的温度范围内测量了 Tm2Ti2O7,并根据平滑热容计算了 0-1900 K 的热力学函数以及氧化物和元素形成的吉布斯能。揭示了 20-320 K 时肖特基异常对热容量的贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Synthesis and Thermodynamic Properties of Thulium Titanate
The temperature stages of crystallization process for thulium titanate of pyrochlore structural type during the heating of hydroxide precursor obtained by reverse precipitation have been studied by DSC/TG, XRD, and electron microscopy. Tm2Ti2O7 has been measured in the temperature range 2–1870 K and thermodynamic functions at 0–1900 K and the Gibbs energy of formation from oxides and elements have been calculated based on smoothed heat capacity. The contribution of the Schottky anomaly at 20–320 K into heat capacity has been revealed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Inorganic Chemistry
化学-无机化学与核化学
CiteScore
3.10
自引率
38.10%
发文量
237
审稿时长
3 months
期刊介绍:
Russian Journal of Inorganic Chemistry is a monthly periodical that covers the following topics of research: the synthesis and properties of inorganic compounds, coordination compounds, physicochemical analysis of inorganic systems, theoretical inorganic chemistry, physical methods of investigation, chemistry of solutions, inorganic materials, and nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


