钠进入γ-石墨烯多层膜:用于金属离子电池阳极的互锁化合物
IF 8.7
1区 化学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
最近实现了γ-石墨烯的批量合成,并证明其具有多层结构,这表明它有可能作为石墨基负极材料的替代品,用于比锂(Li)重的金属。事实上,正如本文通过精确的电子结构计算所报告的那样,其每个亚纳米级大小的规则孔隙都具有容纳单个钠(Na)离子的最佳环境。我们的研究表明,石墨烯/Na 离子耦合在金属-单层相互作用和平衡距离方面模仿了石墨烯/Li 离子的耦合。更重要的是,与石墨相比,我们证明了石墨烯与 Na 的插层化合物在热力学上是稳定的,其最佳存储容量为 372 mAh-g-1。这些发现,加上Na插层时有限的晶体结构扩展、低金属扩散阻力和高导电性,为开发新型石墨基阳极用于高效钠离子电池铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
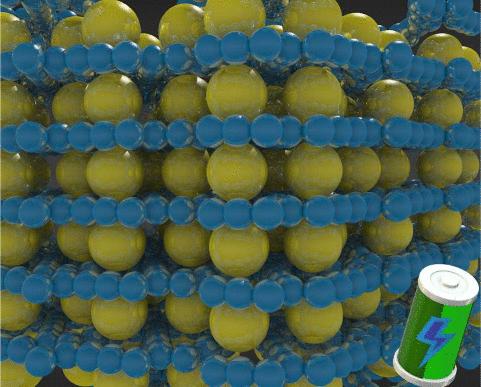
Sodium into γ-Graphyne Multilayers: An Intercalation Compound for Anodes in Metal-Ion Batteries
The bulk synthesis of γ-graphyne has been recently achieved and evidenced a multilayered structure, which suggests its potential exploitation as a substitute of graphite-based anode materials for metals heavier than lithium (Li). In fact, each of its regular pores of sub-nanometric size features an optimal environment for hosting a single sodium (Na) ion, as reported here by means of accurate electronic structure calculations. We show that the graphyne/Na ion coupling mimics that found on the graphene/Li ion in terms of metal-single layer interaction and equilibrium distance. More importantly, in contrast to what is found for graphite, we demonstrate that graphyne intercalation compounds with Na are thermodynamically stable and feature an optimal storage capacity of 372 mAh·g–1. These findings, together with a limited crystal structure expansion upon Na intercalation, a low metal diffusion barrier as well as high electrical conductivity, pave the way to the development of novel graphyne-based anodes for efficient Na-ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Materials Letters
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
14.60
自引率
3.50%
发文量
261
期刊介绍:
ACS Materials Letters is a journal that publishes high-quality and urgent papers at the forefront of fundamental and applied research in the field of materials science. It aims to bridge the gap between materials and other disciplines such as chemistry, engineering, and biology. The journal encourages multidisciplinary and innovative research that addresses global challenges. Papers submitted to ACS Materials Letters should clearly demonstrate the need for rapid disclosure of key results. The journal is interested in various areas including the design, synthesis, characterization, and evaluation of emerging materials, understanding the relationships between structure, property, and performance, as well as developing materials for applications in energy, environment, biomedical, electronics, and catalysis. The journal has a 2-year impact factor of 11.4 and is dedicated to publishing transformative materials research with fast processing times. The editors and staff of ACS Materials Letters actively participate in major scientific conferences and engage closely with readers and authors. The journal also maintains an active presence on social media to provide authors with greater visibility.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


