佩拉米韦从二水合物到三水合物的多态转变机制和晶体习性控制研究
IF 3.4
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
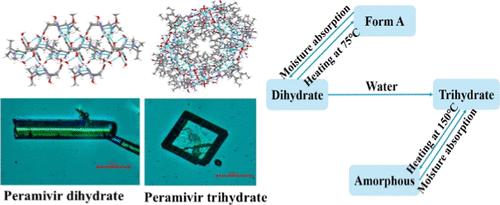
培拉米韦是一种治疗甲型和乙型流感的有效抗病毒药物,以三水合物形式作为固体制剂。然而,在生产过程中仍有少量二水合物存在于少数生产批次中。针对这一问题,本研究成功获得了一种新的二水单晶结构、一种超越以往报道的更高质量的三水单晶结构,以及一种被命名为 A 型的新型无水形态。研究人员对二水培拉米韦和三水培拉米韦的晶体结构、晶体习性、热分析、解溶行为、溶解度、Hirshfeld Surfaces 分析和能量框架进行了综合比较,以了解水分子的结合和脱落过程。三种晶体形态和无定形形态之间的转化过程表明,三水合物形态表现出最大的稳定性。对多晶型转化机理的研究表明,二水合物晶体最初析出,随后溶解,然后在结晶过程中转化为三水合物晶体。转化率与搅拌速度密切相关,提高搅拌速度会加速溶解和晶体转化,从而缩短结晶时间。当搅拌速度从 50 转/分提高到 300 转/分时,从二水合物到三水合物的结晶时间从 10 小时缩短到 5.5 小时。佩拉米韦二水合物(长杆状)和三水合物(四方体状)的晶体习性对比鲜明,这也为快速估算混合晶体提供了依据。这些研究使结晶过程变得稳定可控,在工业生产中能在更短的时间内获得纯净的三水合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study of the Polymorphic Transformation Mechanism and Crystal Habits Control of Peramivir from Dihydrate to Trihydrate
Peramivir is an effective antiviral drug for treating influenza A and B with the trihydrate form as the solid serving. However, a small amount of dihydrate still exists during the manufacturing process in a few production batches. Focusing on the problem, this study successfully obtained a new dihydrate single crystal structure, a higher-quality trihydrate single crystal structure surpassing previously reported findings, and a novel anhydrous form designated as form A. A comprehensive comparison of the crystal structure, crystal habits, thermal analysis, desolvation behavior, solubility, Hirshfeld Surfaces analysis, and energy frameworks was studied between peramivir dihydrate and trihydrate to understand the processes of water molecules binding on and off. The transformation process among the three crystal forms and the amorphous form revealed that the trihydrate form exhibits the most stability. The study of the polymorphic transformation mechanism revealed that dihydrate crystals precipitate initially, dissolve thereafter, and then transform into trihydrate crystals during crystallization. The conversion rate was related strongly to the stirring speed, and increasing the stirring speed accelerated both dissolution and crystal transformation, resulting in a reduction in the duration of crystallization. The crystallization time from dihydrate to trihydrate was shortened from 10 to 5.5 h when the stirring speed was increased from 50 to 300 rpm. The contrasting crystal habits of peramivir dihydrate (long rod-shaped) and trihydrate (tetragonal-shaped) also provide a quick estimate of mixed crystals. These studies made the crystal process stable and controlled, and pure trihydrate was obtained in a shorter time in industrial production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Crystal Growth & Design
化学-材料科学:综合
CiteScore
6.30
自引率
10.50%
发文量
650
审稿时长
1.9 months
期刊介绍:
The aim of Crystal Growth & Design is to stimulate crossfertilization of knowledge among scientists and engineers working in the fields of crystal growth, crystal engineering, and the industrial application of crystalline materials.
Crystal Growth & Design publishes theoretical and experimental studies of the physical, chemical, and biological phenomena and processes related to the design, growth, and application of crystalline materials. Synergistic approaches originating from different disciplines and technologies and integrating the fields of crystal growth, crystal engineering, intermolecular interactions, and industrial application are encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


