Co(II)/Oxone 氧化系统通过 C-N 裂解仲酰胺以获得伯酰胺
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
仲酰胺的 C-N 键的裂解可以为获得伯酰胺提供替代途径;然而,由于酰胺的抗氧化性,这一策略仍然具有挑战性。在此,我们采用了高级氧化工艺(AOPs)之一的钴(II)/Oxone催化系统,使其可以断裂各种仲(磺)酰胺的强C-N键,尤其是那些带有贫电子或正交取代的N-烯的仲酰胺,从而得到理想的伯(磺)酰胺。对照实验表明,N-芳基酰胺初始 N-H 氧化的主要活性物种可能不是钴/过硫酸盐(Co/PMS)体系中一般认为的过硫酸盐阴离子自由基,而是所提出的高价钴-氧中间体。在 N-烷基化仲酰胺的情况下,α-C-H 键而不是 N-H 键应首先被活性自由基和高价钴氧物种氧化。这项工作不仅建立了一种利用易得的环保试剂以低成本去除仲酰胺中 N-取代基的有效方法,还展示了进一步的合成应用,并为环境修复中基于金属的 AOPs 的中间体提供了更多启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
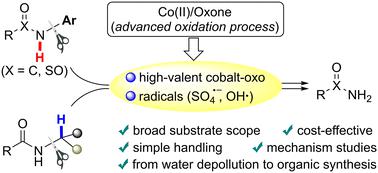
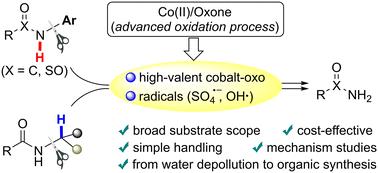
C–N cleavage of secondary amide to access primary amide by a Co(ii)/Oxone oxidation system†
Cleavage of the C–N bond of a secondary amide could provide alternative access to primary amides; however, this strategy remains challenging due to oxidation resistance of the amide. Herein, we employed the cobalt(ii)/Oxone catalytic system, one of the advanced oxidation processes (AOPs), to make it available to break the strong C–N bond of various secondary (sulfon)amides, especially those bearing electron-poor or ortho-substituted N-arenes, en route to desirable primary (sulfon)amides. Control experiments showed that it was probably not the generally-considered persulfate anion radical in the cobalt/peroxymonosulfate (Co/PMS) system but the proposed high-valent cobalt-oxo intermediate that should be the major active species for the initial N–H oxidation of N-aryl amides. In the case of N-alkylated secondary amides, the α-C–H bond, rather than the N–H bond, should be oxidized first by both the reactive radicals and high-valent cobalt-oxo species. This work not only establishes an efficient method for removing the N-substituents of secondary amides at low cost, with readily available and eco-friendly reagents, but also demonstrates further synthetic application and provides more insight into intermediates for metal-based AOPs in environmental remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


