伏瓦来烯醇 A 的合成研究:通过利用 C2 对称性,以不对称方式获得全功能环庚烷框架
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们实现了对映选择性合成天然三萜类化合物伏瓦来烯醇 A 的全官能化环庚烷核心,该核心表现出假 C2 对称性。获得环庚酮核心的关键反应是酶法对映体选择性去对称化(EED)、不对称甲烯丙基化以及环丙烷杂交中间体的还原开环反应。通过一种独特的 "MPV"(Meerwein-Ponndorf-Verley)型环氧化物还原开环反应和其他合成转化,进一步的合成操作得到了目标分子的两个完全官能化的环庚烷框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
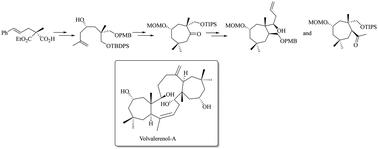
Synthetic studies towards volvalerenol A: access to a fully functionalized cycloheptane framework in an asymmetric fashion through the exploitation of C2-symmetry
The enantioselective synthesis of a fully functionalized cycloheptane core of the naturally occurring triterpenoid volvalerenol A, exhibiting pseudo-C2 symmetry, was achieved. Enantioselective enzymatic desymmetrization (EED), asymmetric methallylation, and reductive ring opening of an cyclopropane overbred intermediate were the key reactions to access the cycloheptanone core. Further synthetic manipulations, via a unique “MPV” (Meerwein–Ponndorf–Verley) type reductive ring-opening of an epoxide and other synthetic transformations, afforded two fully functionalized cycloheptane frameworks of the target molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


