通过干扰 MDR1/P-gp 和 miR 298 之间的串扰,靶向介孔二氧化硅纳米粒子系统性地输送胸腺醌和多柔比星可增敏多柔比星耐药乳腺癌
IF 5.4
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
摘要
乳腺癌的多药耐药性(MDR)仍然是成功化疗的一大障碍。随着临床前研究方法的改进,抗癌药物的种类越来越多,治疗药物组合的增长也是前所未有的。单药治疗的恶性肿瘤往往无法限制癌症的发展,从而导致耐药性癌症的产生,因此,与单药疗法相比,组合疗法具有极大的优势。然而,在选择药物组合时,需要有足够的初步证据来证明其协同效应。化疗药物耐药的基本机制与膜外排泵的过度表达、药物靶点的变化和药物代谢的增加有关。不幸的是,药物很难克服自身或其他不同药物作用产生的耐药性。在这种情况下,我们在本文中报告了一种简单的给药系统,该系统可将胸腺醌(TQ)和多柔比星(DOX)这两种药物共囊化并在细胞内协同给药,从而使对 DOX 产生抗药性的 MDA MB231 细胞系(231 R)恢复敏感。我们实验室开发的 231 R 细胞系显示 ATP 结合盒(ABC)转运体 P-gp1/MDR-1 表达增强,miR-298 表达下降。本递送系统基于胺功能化介孔二氧化硅纳米颗粒(MSNs),其中侧链胺功能基团用于与 TQ 的羰基反应,作为原药系统(TQ-MSN)同时释放 TQ 和 DOX。随后,通过简单的扩散方法将 DOX 包封到上述 TQ-MSN 中。含有药物的 MSN 进一步包覆了透明质酸共轭的 PEG-PLGA 聚合物(HA@TQ-DOX-MSN)。这种简单的纳米策略干扰了 MDR-1/miR-298 的交叉对话,从而大大减少了药物从细胞中的外流,突出了一种基于纳米技术的组合给药方法在控制乳腺癌化疗耐药性方面的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
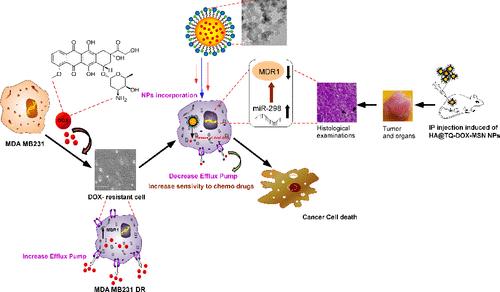
Systemic Codelivery of Thymoquinone and Doxorubicin by Targeted Mesoporous Silica Nanoparticle Sensitizes Doxorubicin-Resistant Breast Cancer by Interfering between the MDR1/P-gp and miR 298 Crosstalk
Multi drug resistance (MDR) in breast carcinoma still poses a significant impairment to successful chemotherapy. As the arsenal of anticancer agents increases with improved preclinical methods, the growth of therapeutic drug combinations is now unprecedented. The malignancies addressed by mono drugs often fail to limit cancer progression, resulting in resistant cancer, thereby offering combinatorial therapies a terrific edge over monodrug regimes. However, the selection of drug combinations required enough preliminary evidence for their synergistic effect. The fundamental mechanisms of MDR to chemotherapeutics are associated with the overexpression of membrane efflux pumps, alternations in drug targets, and increased drug metabolism. Unfortunately, it is very difficult for drugs to overcome resistance produced on their own or by another different drug action. In this context, herein, we report a simple delivery system for coencapsulation and intracellular codelivery of dual-drug thymoquinone (TQ) and doxorubicin (DOX) to resensitize DOX-resistant MDA MB231 cell line (231 R). The 231 R cell line developed in our lab showed an enhanced expression of the ATP-binding cassette (ABC) transporters P-gp1/MDR-1 and a declined miR-298 expression. The present delivery system is based on amine-functionalized mesoporous silica nanoparticles (MSNs), in which the side chain amine functional group was used to react with the carbonyl group of TQ, which acts as a pro-drug system (TQ-MSN) to release TQ and DOX simultaneously. DOX was encapsulated later into the above TQ-MSN by a simple diffusion method. The drugs containing MSNs were further coated with a hyaluronic acid-conjugated PEG–PLGA polymer (HA@TQ-DOX-MSN). This simple nanostrategy interferes with the MDR-1/miR-298 cross-talk, thereby allowing a significant reduction in drug efflux from the cell and highlighting a promising nanotechnology-based combinatorial delivery approach in managing breast cancer chemoresistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Biomaterials Science & Engineering
Materials Science-Biomaterials
CiteScore
10.30
自引率
3.40%
发文量
413
期刊介绍:
ACS Biomaterials Science & Engineering is the leading journal in the field of biomaterials, serving as an international forum for publishing cutting-edge research and innovative ideas on a broad range of topics:
Applications and Health – implantable tissues and devices, prosthesis, health risks, toxicology
Bio-interactions and Bio-compatibility – material-biology interactions, chemical/morphological/structural communication, mechanobiology, signaling and biological responses, immuno-engineering, calcification, coatings, corrosion and degradation of biomaterials and devices, biophysical regulation of cell functions
Characterization, Synthesis, and Modification – new biomaterials, bioinspired and biomimetic approaches to biomaterials, exploiting structural hierarchy and architectural control, combinatorial strategies for biomaterials discovery, genetic biomaterials design, synthetic biology, new composite systems, bionics, polymer synthesis
Controlled Release and Delivery Systems – biomaterial-based drug and gene delivery, bio-responsive delivery of regulatory molecules, pharmaceutical engineering
Healthcare Advances – clinical translation, regulatory issues, patient safety, emerging trends
Imaging and Diagnostics – imaging agents and probes, theranostics, biosensors, monitoring
Manufacturing and Technology – 3D printing, inks, organ-on-a-chip, bioreactor/perfusion systems, microdevices, BioMEMS, optics and electronics interfaces with biomaterials, systems integration
Modeling and Informatics Tools – scaling methods to guide biomaterial design, predictive algorithms for structure-function, biomechanics, integrating bioinformatics with biomaterials discovery, metabolomics in the context of biomaterials
Tissue Engineering and Regenerative Medicine – basic and applied studies, cell therapies, scaffolds, vascularization, bioartificial organs, transplantation and functionality, cellular agriculture
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


