开发用于合成 Janus 激酶抑制剂 GDC-9918 的稳健 Pd 催化 C-S 偶联反应
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
原位形成的(Xantphos)钯氧化加成络合物的开发使功能化芳基溴与 2-巯基乙醇的稳健、可扩展的 C-S 偶联成为可能,最终实现了千克级 Janus 激酶抑制剂 GDC-9918 的合成。研究发现,在 2-巯基乙醇和钯催化剂存在的大多数反应条件下,一种不溶解、催化活性差的 [12] 金属冠-6 钯(II)复合物 [Pd6(μ2-SCH2CH2OH)12]会迅速形成,在我们的第一代路线中,需要高达 12 mol % 的钯才能实现完全转化。为了尽量减少 [12]metallacrown-6 钯(II)络合物的形成,我们开发了第二代工艺,并将催化剂载量降至 2 mol % Pd。可溶性钯清除剂 PIX 能有效地将钯清除到百万分之 5。在钨酸钠催化下,生成的硫醚被顺利氧化,得到了粗制的 GDC-9918,经重结晶后得到了所需的多晶型,并被微粉化成粒度分布合适的颗粒,可作为哮喘的吸入治疗剂进行开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
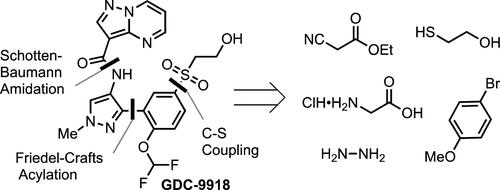
Development of a Robust Pd-Catalyzed C–S Coupling for the Synthesis of Janus Kinase Inhibitor GDC-9918
The development of an in situ formed (Xantphos)Pd oxidative addition complex made possible a robust, scalable C–S coupling of a functionalized aryl bromide with 2-mercaptoethanol that ultimately enabled the synthesis of Janus Kinase inhibitor GDC-9918 on a kilogram scale. An insoluble, catalytically inactive, [12]metallacrown-6 palladium(II) complex, [Pd6(μ2-SCH2CH2OH)12], was found to form quickly under most reaction conditions in the presence of 2-mercaptoethanol and a Pd catalyst and required up to 12 mol % Pd for full conversion in our generation route. A second-generation process was developed to minimize the formation of the [12]metallacrown-6 palladium(II) complex and enabled the decrease in catalyst loading to 2 mol % Pd. A soluble Pd scavenger, PIX, effectively removed Pd to <5 ppm. Catalytic sodium tungstate oxidation of the resulting thioether smoothly provided crude GDC-9918 that was recrystallized to the desired polymorph and micronized to a particle size distribution suitable for development as an inhalable treatment for asthma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


