通过协同催化选择性 C-C 键裂解和随后的烯烃底物 Silylperoxylation 来延长碳链
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过共催化选择性 C-C 键裂解,然后对烯烃底物进行硅烷过氧化反应,生成了一系列烷基硅基过氧化物,而且产量和选择性都很好。通过过渡金属催化裂解生成的烷基硅基过氧化物并随后与偶联剂官能化,可进一步转化生成一系列二羰基化合物。这种两步转化以一锅方式进行,提高了这种方法的实用性。为了解释烯烃底物的 C-C 键裂解和随后的硅基过氧化反应,提出了一种合理的反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
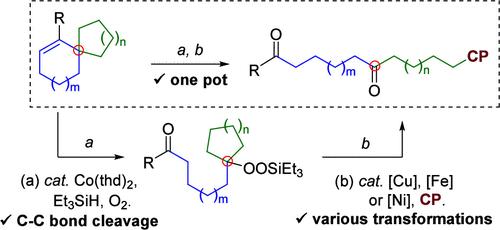
Carbon-Chain Elongation by Co-Catalyzed Selective C–C Bond Cleavage and Subsequent Silylperoxylation of Alkene Substrates
A Co-catalyzed selective C–C bond cleavage followed by the silylperoxylation of alkene substrates generated a series of alkylsilyl peroxides with good yields and selectivities. Further transformation through transition-metal-catalyzed cleavage of the resulting alkylsilyl peroxides and subsequent functionalization with coupling partners could generate a series of dicarbonyl compounds. This two-step transformation was carried out in a one-pot manner, which enhances the practicability of this approach. A plausible reaction mechanism was proposed to interpret the C–C bond cleavage and subsequent silylperoxylation of the alkene substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


