解密 C60 缓冲 Cu/SiO2 催化剂在二甲基甲酰胺转化为乙二醇过程中的催化机理
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
乙二醇和乙醇酸甲酯都可以通过草酸二甲酯加氢合成,它们都是重要的化学原料,了解这些加氢反应的机理细节对于设计高性能催化剂至关重要。在这项工作中,我们采用了精心构建的模型簇催化剂,包括 Cu4、C60Cu、C60Cu3 和 C60Cu4,在质谱、光电子能谱、密度泛函理论计算和分子动力学模拟的共同努力下,模拟了广泛使用的 Cu/SiO2 催化剂和最近发现的 C60 缓冲 Cu/SiO2 催化剂。我们探索了模型簇催化剂催化草酸二甲酯加氢反应的催化机理,并发现了通过改变铜簇尺寸来调节产物选择性的可能性。我们的研究结果揭示了 C60 在促进催化反应中的重要几何和电子效应,它通过向吸附的铜可逆地接受和捐赠电子(即充当电子缓冲器),导致在氢化反应的不同步骤中交替形成 Cu0 和 Cuδ+ 位点。这些氧化态变化对于氢化和稳定反应中间产物至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
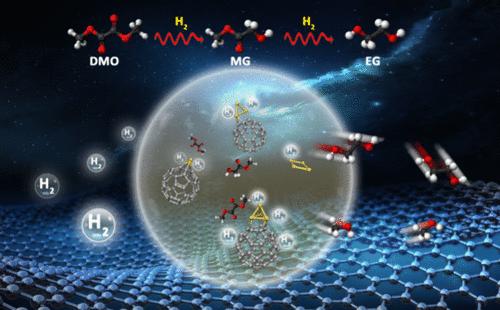
Disentangling the Catalytic Mechanisms of C60-Buffered Cu/SiO2 Catalyst for DMO-to-EG Conversion
Both ethylene glycol and methyl glycolate, which can be synthesized by hydrogenating dimethyl oxalate, are important chemical feedstocks, and understanding the mechanistic details underlying those hydrogenation reactions is of pivotal importance for the design of high-performance catalysts. In this work, we employ carefully constructed model cluster catalysts, including Cu4, C60Cu, C60Cu3, and C60Cu4, to mimic the widely utilized Cu/SiO2 catalyst and the recently discovered C60-buffered Cu/SiO2 catalyst based on joint efforts from mass spectrometry, photoelectron spectroscopy, density functional theory calculations, and molecular dynamics simulations. We explored the catalytic mechanisms of the hydrogenation of dimethyl oxalate catalyzed by our model cluster catalysts and uncovered the possibility to tune the product selectively by changing the copper cluster size. Our results reveal important geometric and electronic effects of C60 in promoting the catalytic reactions by reversibly accepting and donating, i.e., serving as an electron buffer, electrons to the adsorbed copper, resulting in the alternating formation of Cu0 and Cuδ+ sites in different steps of the hydrogenation reactions. These oxidation state changes are crucial for the hydrogenation and stabilization of the reaction intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


