用于可扩展和高效二氧化碳电催化还原的氟调谐碳基镍单原子催化剂
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
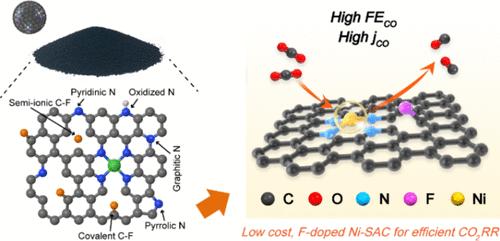
电催化二氧化碳还原技术因其在减缓二氧化碳排放和生产燃料方面的潜在应用而备受关注。然而,相关催化技术的推广仍面临一些挑战,包括催化材料成本高、选择性低、维持理想选择性的电流密度小等。在本研究中,通过聚合物辅助热解,将氟(F)原子引入到掺杂 N 的碳支撑单镍(Ni)原子催化剂中。这种方法不仅能以低成本、可持续的方式保持镍的高原子利用效率,还能通过掺杂 F 有效地操纵活性 Ni-N4 位点的电子结构。此外,还通过调整氟源控制 F 状态(包括共价态和半离子态)进一步优化了催化剂。因此,这种具有独特结构的催化剂在 CO2 到 CO 的转化过程中表现出了相当的电催化性能,在宽电位范围内的法拉第效率(FE)超过 99%,在 -1.16 V 与可逆氢电极(RHE)的相对电压下,CO 演化率高达 9.5 × 104 h-1。它还能提供 400 mA cm-2 的实用电流,同时保持超过 95% 的 CO FE。实验分析结合密度泛函理论(DFT)计算还表明,掺杂 F 改变了 Ni-N4 中心位点的电子构型。这种改变降低了二氧化碳活化的能量障碍,从而促进了关键的 *COOH 中间体的产生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fluorine-Tuned Carbon-Based Nickel Single-Atom Catalysts for Scalable and Highly Efficient CO2 Electrocatalytic Reduction
Electrocatalytic CO2 reduction is garnering significant interest due to its potential applications in mitigating CO2 and producing fuel. However, the scaling up of related catalysis is still hindered by several challenges, including the cost of the catalytic materials, low selectivity, small current densities to maintain desirable selectivity. In this study, Fluorine (F) atoms were introduced into an N-doped carbon-supported single nickel (Ni) atom catalyst via facile polymer-assisted pyrolysis. This method not only maintains the high atom utilization efficiency of Ni in a cost-effective and sustainable manner but also effectively manipulates the electronic structure of the active Ni–N4 site through F doping. The catalyst has also been further optimized by controlling the F states, including convalent and semi-ionic states, by adjusting the fluorine sources involved. Consequently, this catalyst with unique structure exhibited comparable electrocatalytic performance for CO2-to-CO conversion, achieving a Faradaic efficiency (FE) of over 99% across a wide potential range and an exceptional CO evolution rate of 9.5 × 104 h–1 at −1.16 V vs reversible hydrogen electrode (RHE). It also delivered a practical current of 400 mA cm–2 while maintaining more than 95% CO FE. Experimental analysis combined with density functional theory (DFT) calculations have also shown that F-doping modifies the electron configuration at the central Ni–N4 sites. This modification lowers the energy barrier for CO2 activation, thereby facilitating the production of the crucial *COOH intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


