利用酶双自组装技术开发 AIEgens 系金纳米粒子,实现癌症特异性光热疗法的放大
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
精确成像和精确治疗是控制胰腺癌进展的关键。然而,目前的胰腺癌治疗方法受到肿瘤特异性和侵入性手术的限制。在此,我们精心设计了一种胰腺癌特异性光热调制器(AuHQ),该调制器以聚集诱导发射(AIE)发光剂系留的金纳米粒子为主,可促进突出的荧光-光声双模成像引导的光热免疫疗法。一旦到达胰腺肿瘤微环境(TME),AuHQ中的肽链Ala-Gly-Phe-Ser-Leu-Pro-Ala-Gly-Cys(AGFSLPAGC)就会被过表达酶Cathepsin E(CTSE)特异性裂解,从而引发AuNPs和AIE发光剂的双重自组装。由酶裂解介导的 AuNPs 聚集可在近红外(NIR)激光照射下增强光热疗法(PTT)、诱导免疫性细胞死亡(ICD)和增强光声成像。同时,通过疏水作用形成的AIE发光原聚集体可产生AIE荧光,从而实现对胰腺癌的实时和特异性荧光成像。此外,将吲哚胺-2,3-二氧合酶1(IDO1)抑制剂与AuHQ联合使用,可以解决免疫抑制性TME对PTT疗效的限制,并发挥其激活全身抗肿瘤免疫的协同潜力。因此,这种精心设计的光otheranostic调制剂AuHQ有助于成像和治疗药物的智能酶促双自组装,为胰腺癌治疗学提供了一种高效、精确的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
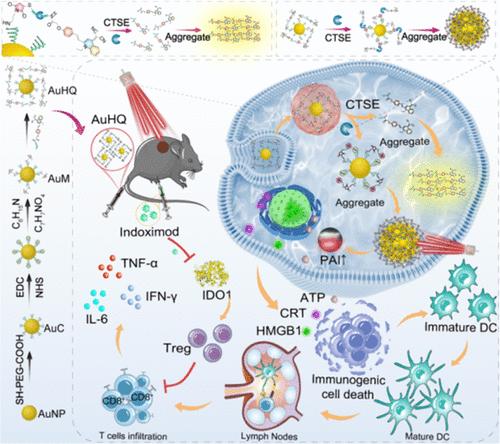
Engineering AIEgens-Tethered Gold Nanoparticles with Enzymatic Dual Self-Assembly for Amplified Cancer-Specific Phototheranostics
Accurate imaging and precise treatment are critical to controlling the progression of pancreatic cancer. However, current approaches for pancreatic cancer theranostics suffer from limitations in tumor specificity and invasive surgery. Herein, a pancreatic cancer-specific phototheranostic modulator (AuHQ) dominated by aggregation-induced emission (AIE) luminogens-tethered gold nanoparticles is meticulously designed to facilitate prominent fluorescence-photoacoustic bimodal imaging-guided photothermal immunotherapy. Once reaching the pancreatic tumor microenvironment (TME), the peptide Ala-Gly-Phe-Ser-Leu-Pro-Ala-Gly-Cys (AGFSLPAGC) linkage within AuHQ can be specifically cleaved by the overexpressed enzyme Cathepsin E (CTSE), triggering the dual self-assembly of AuNPs and AIE luminogens. The aggregation of AuNPs mediated by enzymatic cleavage results in potentiated photothermal therapy (PTT) under near-infrared (NIR) laser irradiation, induced immunogenic cell death (ICD), and enhanced photoacoustic imaging. Simultaneously, AIE luminogen aggregates formed by hydrophobic interaction can generate AIE fluorescence, enabling real-time and specific fluorescence imaging of pancreatic cancer. Furthermore, coadministration of an indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor with AuHQ can address the limitations of PTT efficacy imposed by the immunosuppressive TME and leverage the synergistic potential to activate systemic antitumor immunity. Thus, this well-designed phototheranostic modulator AuHQ facilitates the intelligent enzymatic dual self-assembly of imaging and therapeutic agents, providing an efficient and precise approach for pancreatic cancer theranostics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


