引导电子转移,从二氧化碳中选择性光生化 C2H6
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
无引导电子转移给选择性光还原二氧化碳(CO2)为 C2 产物带来了挑战。我们通过原位化学封装在光催化剂中构建了连续的组分间和组分内电场。双串联电场可促进电荷分离,并将光生电子准确转移到 Cu2+-Cu+ 位点,实现 C-C 耦合。我们对电子传输进行了跟踪,观察到电子在接触的异质结构原子、配体碳原子和 Cu2+-Cu+ 中心之间的定向迁移。合成的光催化剂在水蒸气中的乙烷(C2H6)生产率高达 16.3 μmol g-1 h-1,对 C2H6 的电子选择性高达 64.4%,电子消耗量稳定在 354.6 μmol g-1 h-1。这代表了二氧化碳光电还原的最佳性能之一。这项研究通过串联内置电场促进了电荷分离并对电子迁移进行了精确控制,为将二氧化碳光还原成高价值化学品开辟了新的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
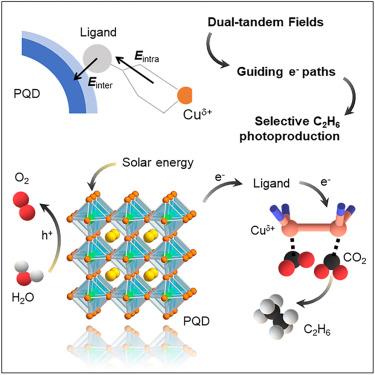

Guiding electron transfer for selective C2H6 photoproduction from CO2
Unguided electron transfer presents challenges for selectively photo-reducing carbon dioxide (CO2) into C2 products. We constructed continuous inter- and intra-component electric fields within photocatalysts by in situ chemical encapsulation. The dual-tandem electric fields facilitate charge separation and transfer photogenerated electrons accurately toward Cu2+-Cu+ sites for C–C coupling. We tracked the electron transport, observing directional electron migration between contacted heterostructure atoms, ligand carbon atoms, and Cu2+-Cu+ centers. The as-synthesized photocatalyst manifests a remarkable ethane (C2H6) production rate of 16.3 μmol g−1 h−1, a high electron selectivity of 64.4% for C2H6, and a stable electron consumption yield of 354.6 μmol g−1 h−1 in water vapor. These represent one of the best performances for CO2 photoreduction. This work promotes charge separation and manages precise control over electron migration via tandem built-in electric fields, opening a new prospect for selective CO2 photoreduction into high-value chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


