候选高压阴极材料 Li2MnP2S6 的合成、电子结构和氧化还原化学性质
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
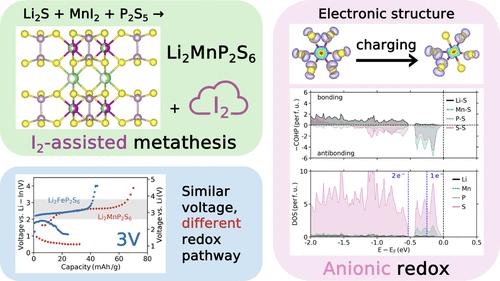
虽然人们为利用硫化物基正极的大容量做出了巨大努力,但对提高其电压的关注却很有限。在此,我们采用新型碘化物辅助合成路线,成功合成了金属硫代磷酸锂 Li2MP2S6(M = Mn、Fe 和 Co),其中 Li2MnP2S6 是一种新型化合物。从 Li2FeP2S6 和 Li2MnP2S6 中电化学提取锂的电压为 3 V,明显高于其他硫化物阴极。尽管电压相似,但这两种材料的氧化还原机制却截然不同。密度泛函理论计算和 X 射线吸收光谱显示,Li2FeP2S6 主要表现出传统的阳离子氧化还原,而 Li2MnP2S6 的氧化还原则涉及大量阴离子氧化还原。我们对 Li2MnP2S6 的分析还可用于解释近期有关其他富锂硫磷酸盐阴极的研究。这项工作介绍了获得硫化物基材料的新合成途径,并揭示了硫代磷酸盐基阴极的高压氧化还原机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, Electronic Structure, and Redox Chemistry of Li2MnP2S6, a Candidate High-Voltage Cathode Material
While significant efforts have been made to harness the large capacity of sulfide-based cathodes, there has been limited focus on increasing their voltage. Here, by a novel iodide-assisted synthesis route, we successfully synthesized lithium metal thiophosphates Li2MP2S6 (M = Mn, Fe, and Co), of which Li2MnP2S6 is a new compound. Electrochemical extraction of Li from Li2FeP2S6 and Li2MnP2S6 was performed at ∼3 V, significantly higher than other sulfide-based cathodes. Despite the similar voltages, these two materials were found to operate by very different redox mechanisms. Density functional theory calculations and X-ray absorption spectroscopy show that while Li2FeP2S6 exhibits mostly traditional cationic redox, Li2MnP2S6 redox involves significant participation of anionic redox. Our analysis of Li2MnP2S6 is also used to contextualize recent work on other Li-rich thiophosphate cathodes. This work introduces a new synthetic route to access sulfide-based materials and sheds insights into the high-voltage redox mechanism in thiophosphate-based cathodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


