通过电子转移途径活化过硫酸盐的掺氮碳材料
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在碳材料中加入氮元素是一种能有效提高其催化活性的策略。本文以三聚氰胺为前驱体,合成了一种富氮碳物质,命名为 CN0.6。这种物质已被证实是一种不含金属的高效催化剂,可活化过一硫酸盐(PMS)。在 25 °C 的温度下,0.05 g/L 的 CN0.6 浓度和 1 mM 的 PMS 足以在短短 4 分钟内实现浓盐酸四环素(TC)的完全降解。催化性能的提高归功于最佳的氮掺杂水平,它提高了吡咯烷酮氮的含量,并引入了 ID/IG 比为 1.02 的额外缺陷。这些因素共同提高了对 PMS 的吸附能力,并产生了更多的活性位点,从而促进了 PMS 的活化。通过一系列分析,包括自由基鉴定、淬火测试和电化学评估,CN0.6/PMS 系统中的非自由基电子转移机制的主导地位得到了证实。通过使用高分辨率液相色谱法和串联质谱法(LC-MS),该研究确定了 TC 的三种潜在降解途径。此外,与三氯乙酸相比,所产生的中间产物毒性更低。这项研究的结果为合成高效的掺氮无金属催化剂提供了一种方法,为降解环境污染物提供了一种前景广阔的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
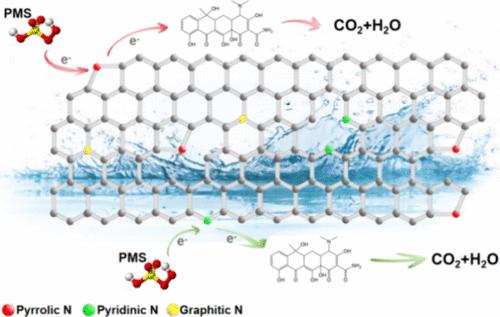
Nitrogen-Doped Carbon Materials for Persulfate Activation via Electron Transfer Pathways
The incorporation of nitrogen into carbon materials is a strategy that effectively boosts their catalytic potency. Herein, a nitrogen-enriched carbon substance, designated as CN0.6, was synthesized from melamine, serving as a precursor. This substance has been established to act as an efficient catalyst devoid of metals for the activation of peroxymonosulfate (PMS). At a temperature of 25 °C, a concentration of 0.05 g/L CN0.6 along with 1 mM PMS suffices to achieve the complete degradation of concentrated tetracycline hydrochloride (TC) in a short period of 4 min. This enhanced catalytic performance is attributed to the optimal level of nitrogen doping, which elevates the pyrrolic nitrogen content and introduces additional defects characterized by an ID/IG ratio of 1.02. These factors collectively augment the adsorptive capacity for PMS and create a greater number of active sites to facilitate its activation. The dominance of a nonradical electron transfer mechanism in the CN0.6/PMS system has been confirmed through a series of analyses, including radical identification, quenching tests, and electrochemical assessments. Employing high-resolution liquid chromatography coupled with tandem mass spectrometry (LC-MS), the investigation identified three potential degradation routes for TC. Furthermore, the intermediates produced are determined to possess reduced toxicity in comparison to TC. The findings of this study offer a approach to the synthesis of highly efficient nitrogen-doped, metal-free catalysts, presenting a promising strategy for the degradation of environmental pollutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


