大肠杆菌通过改造的氮酶组装途径合成氨
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于氮酶对农业、能源和环境的深远影响,人们一直在积极寻求氮酶的异源表达。然而,从非氮养宿主中分离出活性双组分、含金属中心的氮酶的工作尚未完成。在此,我们报告了通过在大肠杆菌中结合来自乙烯氮芽胞杆菌(Azotobacter vinelandii)和乙酰甲烷菌(Methanosarcina acetivorans)的基因异源合成活性钼氮酶的情况。金属、活性和电子顺磁共振分析表明了纯化的氮酶中金属中心的完整性;而生长、纳米级二次离子质谱分析和核磁共振实验表明了大肠杆菌表达菌株的重氮生长和 15N 富集,以及氨转运体缺失后细胞外氨的积累,氨转运体允许将由此产生的氮并入非重氮大肠杆菌菌株的细胞质中。因此,这项研究提供了一个重要的原型系统,可以对其进行优化/改造,使氮酶将来能够进行转基因表达和生物技术改造。本文章由计算机程序翻译,如有差异,请以英文原文为准。

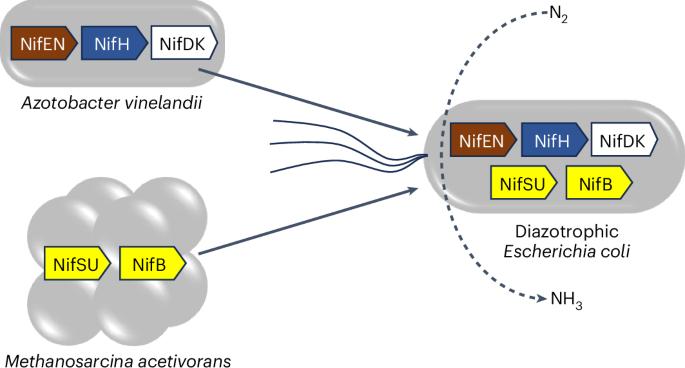
Ammonia synthesis via an engineered nitrogenase assembly pathway in Escherichia coli
Heterologous expression of nitrogenase has been actively pursued because of the far-reaching impact of this enzyme on agriculture, energy and the environment. However, isolation of an active two-component, metallocentre-containing nitrogenase from a non-diazotrophic host has yet to be accomplished. Here we report the heterologous synthesis of an active molybdenum-nitrogenase by combining genes from Azotobacter vinelandii and Methanosarcina acetivorans in Escherichia coli. Metal, activity and electron paramagnetic resonance analyses demonstrate the integrity of the metallocentres in the purified nitrogenase enzyme; whereas growth, nanoscale secondary ion mass spectrometry and nuclear magnetic resonance experiments illustrate diazotrophic growth and 15N enrichment by the E. coli expression strain, and accumulation of extracellular ammonia upon deletion of the ammonia transporter that permits incorporation of thus-generated nitrogen into the cellular mass of a non-diazotrophic E. coli strain. As such, this study provides a crucial prototype system that could be optimized/modified to enable future transgenic expression and biotechnological adaptations of nitrogenase. Heterologous expression of an active, metallocentre-containing nitrogenase in a non-diazotrophic host is challenging. Now, the heterologous biosynthetic pathway of Mo-nitrogenase is pieced together in Escherichia coli using genes from Azotobacter vinelandii and Methanosarcina acetivorans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


