在无催化剂条件下一步法连续合成融合和非融合多取代二氢吡啶衍生物
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报告采用了一种单锅、连续、三组分的方法,通过二胺或氨基苯酚、1,1-二甲基-2-硝基乙烯和 α,β-不饱和芳香酮在乙腈中的缩合反应,在回流条件下合成多取代二氢吡啶。在较短的反应时间内,制备出了一系列融合和非融合的多取代二氢吡啶,收率高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
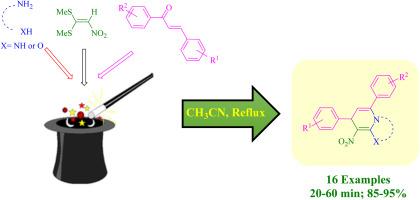
One-pot sequential synthesis of fused and non-fused poly-substituted dihydropyridine derivatives under catalyst-free conditions
A one-pot, sequential, three-component procedure is reported for the synthesis of poly-substituted dihydropyridines via the condensation reaction of diamines or aminophenoles, 1,1-bismethylmethio-2-nitroethylene and α,β-unsaturated aromatic ketones in acetonitrile under reflux conditions. A range of fused and non-fused poly-substituted dihydropyridines were prepared in good to high yields with short reaction times.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


