新型柯萨奇病毒 A9 荚膜粘合剂的 SAR 分析
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
肠道病毒感染在人类中很常见,但目前还没有获得批准的抗病毒治疗方法。在这项研究中,我们集中研究了肠道病毒 B(EV-B)之一,即柯萨奇病毒 A9(CVA9)的抑制作用,结合使用了药物化学、病毒抑制试验、低温电子显微镜结构测定和分子建模等方法,确定了一类新型 N-苯基苄胺的结构活性关系。在新合成的 29 个化合物中,10 个化合物的半数最大有效浓度(EC50)值在 0.64-10.46 μM 之间,其中 7 个化合物的 50%细胞毒性浓度(CC50)值高于 200 μM。此外,这一系列新化合物还显示出良好的理化特性,并通过稳定囊壳发挥作用,防止囊壳扩张和随后的基因组释放。本文章由计算机程序翻译,如有差异,请以英文原文为准。
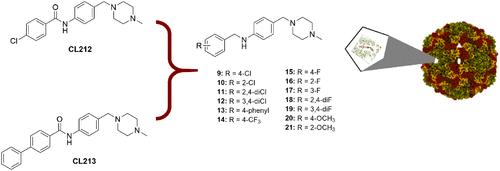
SAR Analysis of Novel Coxsackie virus A9 Capsid Binders
Enterovirus infections are common in humans, yet there are no approved antiviral treatments. In this study we concentrated on inhibition of one of the Enterovirus B (EV-B), namely Coxsackievirus A9 (CVA9), using a combination of medicinal chemistry, virus inhibition assays, structure determination from cryogenic electron microscopy and molecular modeling, to determine the structure activity relationships for a promising class of novel N-phenylbenzylamines. Of the new 29 compounds synthesized, 10 had half maximal effective concentration (EC50) values between 0.64–10.46 μM, and of these, 7 had 50% cytotoxicity concentration (CC50) values higher than 200 μM. In addition, this new series of compounds showed promising physicochemical properties and act through capsid stabilization, preventing capsid expansion and subsequent release of the genome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


