拟肽类似物通过阻断胰蛋白酶 L 的功能有效抑制 SARS-CoV-2
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
Cathepsin L(CatL)是一种重要的蛋白酶,能裂解严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)的尖峰蛋白,增强病毒进入细胞的能力,因此是一种很有前景的严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)抗病毒药物靶点。我们发现了一种三肽醛候选物 D1-1,它在 Vero E6 细胞中对 SARS-CoV-2 有抑制作用。蛋白酶筛选分析和蛋白质下拉实验证明了 D1-1 与 CatL 的直接结合。在分子对接的指导下,我们合成了 72 个类似物。通过分析这些抑制剂的结构-活性关系,我们开发出了 D6 系列。其中,D6-3 是最有效的 CatL 抑制剂(IC50 = 0.27 nM,EC50 = 0.26 μM)。D6-3有效地阻断了CatL的功能,大大阻碍了SARS-CoV-2伪病毒进入细胞。我们的工作提出了靶向和抑制 CatL 的新型化合物,为开发 SARS-CoV-2 抗病毒药物提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
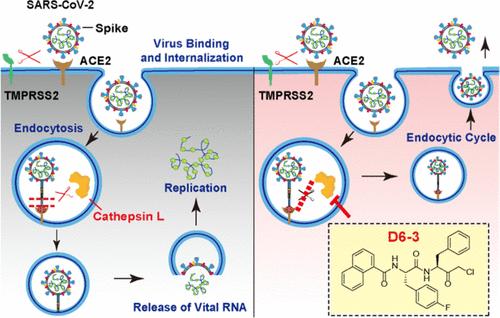
Peptidomimetic Analogues Act as Effective Inhibitors against SARS-CoV-2 by Blocking the Function of Cathepsin L
Cathepsin L (CatL) is a promising antiviral drug target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as an important protease for cleaving the SARS-CoV-2 spike protein and enhancing viral entry to cells. We identified a tripeptide aldehyde candidate, D1–1, which exhibited inhibitory effects against SARS-CoV-2 in Vero E6 cells. The protease screening analysis and protein pull-down assays demonstrated the direct binding of D1–1 to CatL. Guided by molecular docking, we synthesized 72 analogues. Upon analyzing the structure–activity relationships of these inhibitors, the D6 series was developed. Among them, D6–3 functioned as the most potent CatL inhibitor (IC50 = 0.27 nM, EC50 = 0.26 μM). D6–3 effectively blocked the CatL function and substantially hindered the entry of the SARS-CoV-2 pseudovirus to cells. Our work presented novel compounds for targeting and inhibiting CatL, offering valuable insights into the development of SARS-CoV-2 antivirals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


