通过 VO(acac)2/希夫碱催化硫化物中间体的不对称氧化作用立体选择性合成马拉利西巴特
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过 VO(acac)2/希夫碱催化苯硫酚手性中间体的不对称氧化,利用具有立体性的 R-亚砜官能团的手性转移效应,实现了马拉利西巴特的立体选择性合成。R- 亚砜中间体经过闭环反应,形成了具有所需立体化学结构的七元环核心结构,最终确保了该药物的优异异构体纯度和合成效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
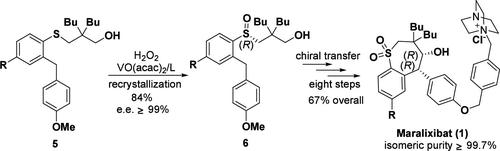
Stereoselective Synthesis of Maralixibat via VO(acac)2/Schiff Base-Catalyzed Asymmetric Oxidation of Its Sulfide Intermediate
The stereoselective synthesis of maralixibat was achieved by harnessing the chiral transferring effect of the stereogenic R-sulfoxide functionality, which was obtained via the VO(acac)2/Schiff base-catalyzed asymmetric oxidation of a phenylthiophenol prochiral intermediate. The R-sulfoxide intermediate underwent a ring closure reaction to form the seven-membered ring core structure with the desired stereochemistry, ultimately ensuring the drug’s exceptional isomeric purity and synthetic efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


