线粒体硫酸化蛋白靶向共价固定化促进高效铜(II)耗竭以加强癌症治疗
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
铜在细胞代谢和氧化应激调节中发挥着重要作用。线粒体铜含量的可视化和控制已被证明是诊断和治疗三阴性乳腺癌(TNBC)的有效策略。然而,开发一种先进的探针来同时可视化和消耗线粒体中的铜仍然是一个巨大的挑战。在此,我们首次报道了线粒体锚定、铜响应和耗竭探针 d-IR-DPA,并评估了其在体内定量观察瘤内铜(II)和抗 TNBC 的潜力。该探针利用线粒体靶向性和亚硫酸化蛋白介导的共价固定特性,不仅能通过比率光声(PA680 nm/PA800 nm)成像定量检测各类肿瘤中的 Cu2+ 水平,还能清除线粒体中的 Cu2+,同时引发氧化应激增加和线粒体膜损伤,最终导致 TNBC 细胞严重凋亡。更值得注意的是,d-IR-DPA 对 Cu2+ 的消耗可改变细胞代谢途径,从氧化磷酸化转变为糖酵解,从而诱导能量剥夺,显著抑制活体小鼠 TNBC 肿瘤的生长。我们的探针可能会为有效治疗 TNBC 及其他铜相关疾病提供有价值的有力手段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
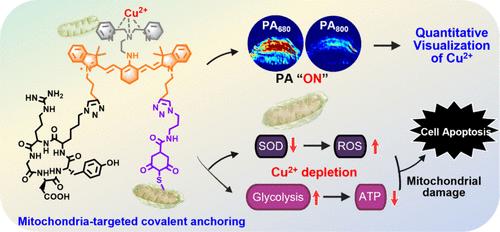
Mitochondrial Sulfenated-Protein-Targeted Covalent Immobilization Boosting Efficient Copper(II) Depletion for Enhanced Cancer Treatment
Copper plays a vital role in cellular metabolism and oxidative stress regulation. Visualizing and controlling the copper level in mitochondrion have been proven to be promising and efficient strategies for the diagnosis and treatment of triple-negative breast cancer (TNBC). However, developing an advanced probe for simultaneous visualization and depletion of mitochondrial copper remains a huge challenge. Herein, we for the first time report a mitochondria-anchorable, copper-responsive, and depleting probe d-IR-DPA and evaluate its potential for quantitative visualization of intratumoral copper(II) and anti-TNBC in vivo. Taking advantage of the mitochondrion-targeting and sulfenated-protein-mediated covalent immobilization characteristics, this probe not only enables the quantitative detection of Cu2+ levels in various types of tumors through ratiometric photoacoustic (PA680 nm/PA800 nm) imaging but also scavenges the mitochondrial Cu2+, simultaneously igniting increased oxidative stress and mitochondrial membrane damage and eventually leading to severe TNBC cell apoptosis. More notably, the depletion of Cu2+ by d-IR-DPA can alter the cellular metabolic pathway from oxidative phosphorylation to glycolysis, inducing energy deprivation and significant suppression of TNBC tumor in living mice. Our probe may provide a valuable and powerful means for the effective treatment of TNBC as well as other copper-associated diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


