抑制阿尔茨海默病模型小鼠的 Ca2+ 通道可松弛周细胞、改善脑血流并减少免疫细胞停滞和缺氧现象
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
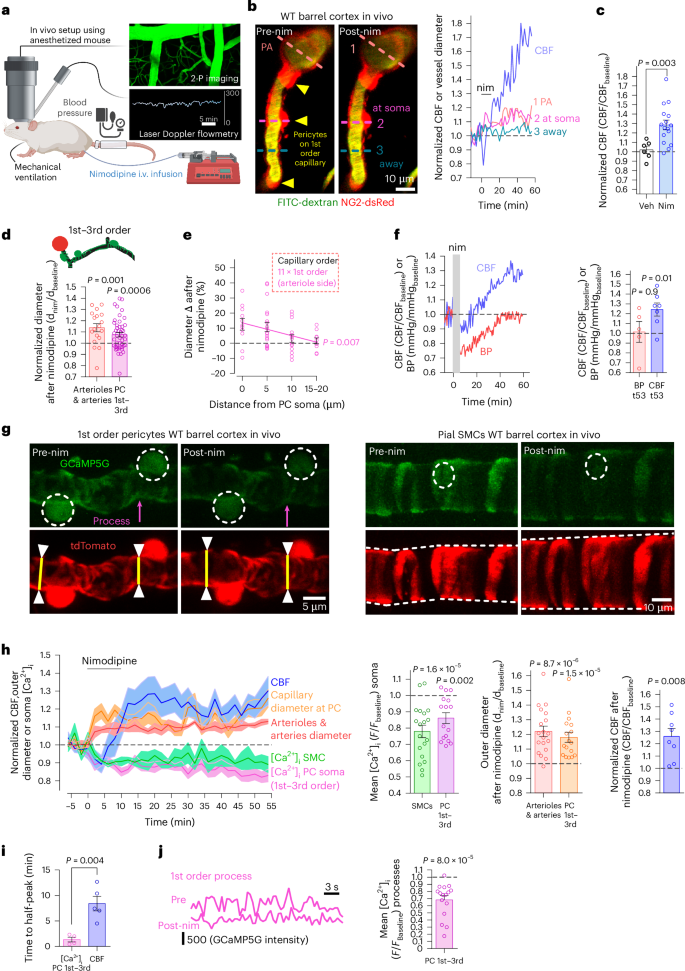
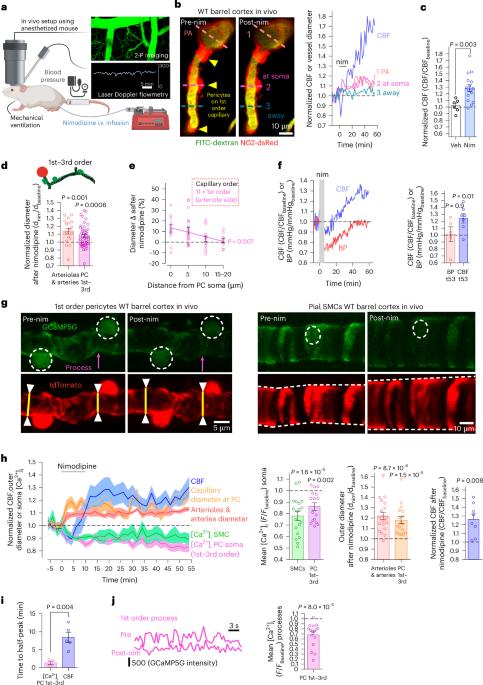
阿尔茨海默病(AD)早期,周细胞会收缩毛细血管,增加毛细血管的水阻力并困住免疫细胞,从而降低脑血流量(CBF)。目前还缺乏治疗方法来减弱包膜细胞介导的AD收缩。在这里,我们利用体内双光子成像激光多普勒和斑点血流测量以及磁共振成像技术,证明了通过 L 型电压门控钙通道(CaVs)进入的 Ca2+ 控制着周细胞的收缩张力。在AD模型小鼠中,我们发现整个毛细血管床的周细胞是免疫活性氧(ROS)诱发和周细胞胞内钙浓度([Ca2+]i)介导的微血管流量下降的主要驱动因素。在疾病进展早期用尼莫地平阻断钙离子通道可改善CBF,减少白细胞在周细胞体节的滞留,并减轻脑缺氧。淀粉样β(Aβ)诱发的人皮质组织周细胞收缩也因 CaV 阻断而大大减少。因此,在注意力缺失症早期降低周细胞[Ca2+]i可能是提高大脑能量供应和认知功能的一种治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhibiting Ca2+ channels in Alzheimer’s disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia
Early in Alzheimer’s disease (AD), pericytes constrict capillaries, increasing their hydraulic resistance and trapping of immune cells and, thus, decreasing cerebral blood flow (CBF). Therapeutic approaches to attenuate pericyte-mediated constriction in AD are lacking. Here, using in vivo two-photon imaging with laser Doppler and speckle flowmetry and magnetic resonance imaging, we show that Ca2+ entry via L-type voltage-gated calcium channels (CaVs) controls the contractile tone of pericytes. In AD model mice, we identifed pericytes throughout the capillary bed as key drivers of an immune reactive oxygen species (ROS)-evoked and pericyte intracellular calcium concentration ([Ca2+]i)-mediated decrease in microvascular flow. Blocking CaVs with nimodipine early in disease progression improved CBF, reduced leukocyte stalling at pericyte somata and attenuated brain hypoxia. Amyloid β (Aβ)-evoked pericyte contraction in human cortical tissue was also greatly reduced by CaV block. Lowering pericyte [Ca2+]i early in AD may, thus, offer a therapeutic strategy to enhance brain energy supply and possibly cognitive function in AD. Early in Alzheimer’s disease (AD), brain blood flow is reduced by pericytes constricting capillaries. Korte et al. show that oral nimodipine can reverse this and decrease brain hypoxia. Blocking capillary constriction is a potential add-on therapy in AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


