铋类药物通过破坏铁稳态使铜绿假单胞菌对多种抗生素敏感
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
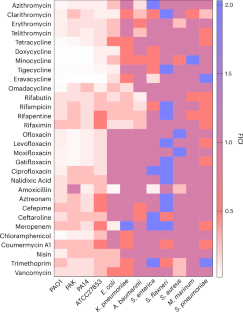
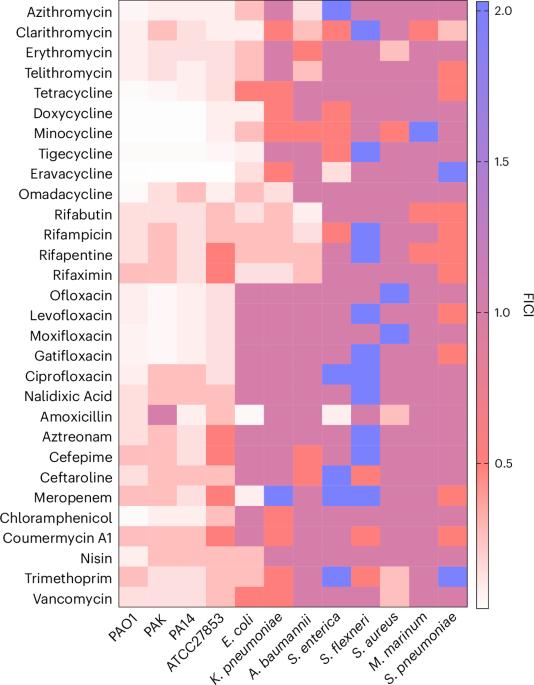
由于抗生素耐药性的快速发展,铜绿假单胞菌感染难以治疗。与开发新的抗生素相比,将已在使用的药物进行协同组合是对抗抗生素耐药性细菌的一种替代方法。在这里,我们证明了以铋为基础的药物(亚水杨酸铋、胶体亚柠檬酸铋)与不同种类的抗生素(四环素类、大环内酯类、喹诺酮类、利福霉素类等)联合使用,可以消灭耐多药的铜绿假单胞菌,并且不会诱发抗生素耐药性的产生。铋能与铜绿假单胞菌的嗜苷酸盐结合,从而破坏铁的平衡。在细胞内,铋通过破坏含铁硫簇的酶,包括呼吸复合物,抑制电子传递链,消散质子动力,并损害外排泵的活性。因此,铋有利于抗生素在细菌体内的蓄积,从而增强了抗生素的疗效。这种联合疗法在体外菌血症模型中显示出强大的抗菌效力和较低的毒性,在体内小鼠肺部感染模型中提高了小鼠的存活率。我们的研究结果凸显了铋类药物与临床常用抗生素联用,重新用于抗击铜绿假单胞菌感染的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bismuth-based drugs sensitize Pseudomonas aeruginosa to multiple antibiotics by disrupting iron homeostasis
Pseudomonas aeruginosa infections are difficult to treat due to rapid development of antibiotic drug resistance. The synergistic combination of already-in-use drugs is an alternative to developing new antibiotics to combat antibiotic-resistant bacteria. Here we demonstrate that bismuth-based drugs (bismuth subsalicylate, colloidal bismuth subcitrate) in combination with different classes of antibiotics (tetracyclines, macrolides, quinolones, rifamycins and so on) can eliminate multidrug-resistant P. aeruginosa and do not induce development of antibiotic resistance. Bismuth disrupts iron homeostasis by binding to P. aeruginosa siderophores. Inside cells, bismuth inhibits the electron transport chain, dissipates the proton motive force and impairs efflux pump activity by disrupting iron–sulfur cluster-containing enzymes, including respiration complexes. As a result, bismuth facilitates antibiotic accumulation inside bacteria, enhancing their efficacy. The combination therapy shows potent antibacterial efficacy and low toxicity in an ex vivo bacteraemia model and increases the survival rate of mice in in vivo mouse lung-infection models. Our findings highlight the potential of bismuth-based drugs to be repurposed to combat P. aeruginosa infections in combination with clinically used antibiotics. Bismuth-based drugs combined with antibiotics enhance efficacy against multidrug-resistant Pseudomonas aeruginosa by disrupting bacterial iron homeostasis and electron transport.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


