致癌 p53 触发 p63 和 p73 液滴的淀粉样聚集
IF 6.2
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
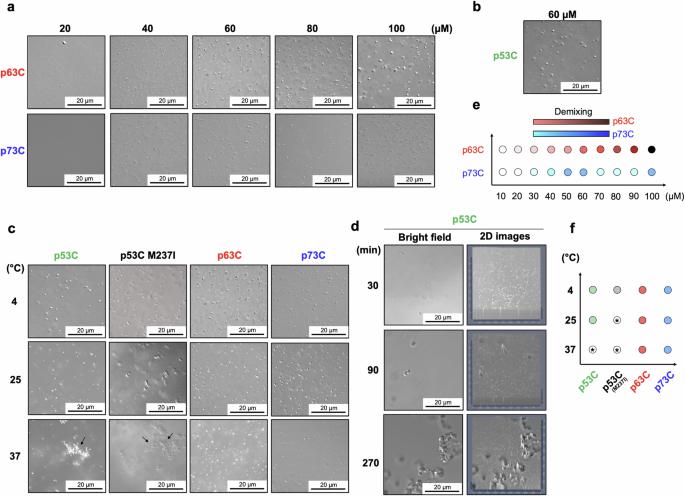
P53 相分离对淀粉样蛋白聚集至关重要,p63 和 p73 在肿瘤中的表达增强。本研究考察了 p53、p63 和 p73 的相行为。我们在这里发现,与 p53 的 DNA 结合结构域(p53C)不同,p63C 和 p73C 会发生相分离,但在生理温度下不会形成淀粉样。野生型和突变型 p53C 在 4°C 时形成液滴,在 37°C 时形成具有淀粉样特性的聚集体。突变体 p53C 促进了 p63C 和 p73C 的淀粉样状态,将它们招募到无膜细胞器中。硫黄素 T 和刚果红的结合、光散射的增加以及圆二色性都支持淀粉样转化。全长突变 p53 和 p63C(或 p73C)共转染显示光漂白后荧光恢复减少。肝素抑制了 p53C 诱导的 p63C 和 p73C 的朊病毒样聚集。这些发现强调了 p53 在引发 p63 和 p73 的淀粉样聚集中的作用,为在癌症治疗中靶向朊病毒样转化开辟了途径。p53 的相分离对其走向淀粉样聚集至关重要,而其旁系形式 p63 和 p73 在肿瘤中的表达增强,但聚集倾向降低。作者在本文中报告了由 p53 介导的 p63 和 p73 的朊病毒样聚集,并概述了肝素可以抑制这一过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oncogenic p53 triggers amyloid aggregation of p63 and p73 liquid droplets
P53 Phase separation is crucial towards amyloid aggregation and p63 and p73 have enhanced expression in tumors. This study examines the phase behaviors of p53, p63, and p73. Here we show that unlike the DNA-binding domain of p53 (p53C), the p63C and p73C undergo phase separation, but do not form amyloids under physiological temperatures. Wild-type and mutant p53C form droplets at 4°C and aggregates at 37 °C with amyloid properties. Mutant p53C promotes amyloid-like states in p63C and p73C, recruiting them into membraneless organelles. Amyloid conversion is supported by thioflavin T and Congo red binding, increased light scattering, and circular dichroism. Full-length mutant p53 and p63C (or p73C) co-transfection shows reduced fluorescence recovery after photobleaching. Heparin inhibits the prion-like aggregation of p63C and p73C induced by p53C. These findings highlight the role of p53 in initiating amyloid aggregation in p63 and p73, opening avenues for targeting prion-like conversion in cancer therapy. Phase separation of p53 is crucial in its progression towards amyloid aggregation, while its paralogous forms p63 and p73 have enhanced expression in tumors but reduced aggregation propensity. Here, the authors report the prion-like aggregation of p63 and p73 mediated by p53 and outline that this process can be inhibited by heparin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


