低频正弦电磁场通过调节 miR-34b-5p/STAC2 促进大鼠骨髓间充质干细胞的成骨分化
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
电磁场已成为治疗骨质疏松症的一种有效方法。然而,其治疗功效的具体机制仍存在争议。在这里,我们证实了 15 Hz 和 0.4-1 mT 低频正弦波电磁场(SEMFs)对大鼠骨髓间充质干细胞(BMSCs)的促骨生成作用。随后的 miRNA 测序发现,在 0.4 mT 和 1 mT SEMFs 刺激组中,miR-34b-5p 均出现下调。为明确 miR-34b-5p 在成骨过程中的作用,分别用 miR-34b-5p 模拟物和抑制剂转染 BMSCs。结果表明,miR-34b-5p模拟物转染抑制成骨分化,而抑制miR-34b-5p则促进BMSCs的成骨分化。利用微型计算机断层扫描、H&E 染色和马森染色进行的体内评估表明,注射 miR-34b-5p 抑制剂可减轻卵巢切除术(OVX)大鼠的骨量损失和骨小梁微结构恶化。进一步的验证表明,miR-34b-5p 是通过调节 STAC2 的表达来发挥其作用的。调节 miR-34b-5p/STAC2 轴可减轻低频 SEMF 对 BMSCs 的促成骨作用。这些研究表明,SEMFs的促成骨作用部分是由于调控了miR-34b-5p/STAC2通路,这为骨质疏松症提供了一种潜在的候选疗法。低频正弦电磁场通过调节miR34b-5p/STAC2轴增强了小鼠的骨生成,为潜在的骨质疏松症治疗提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
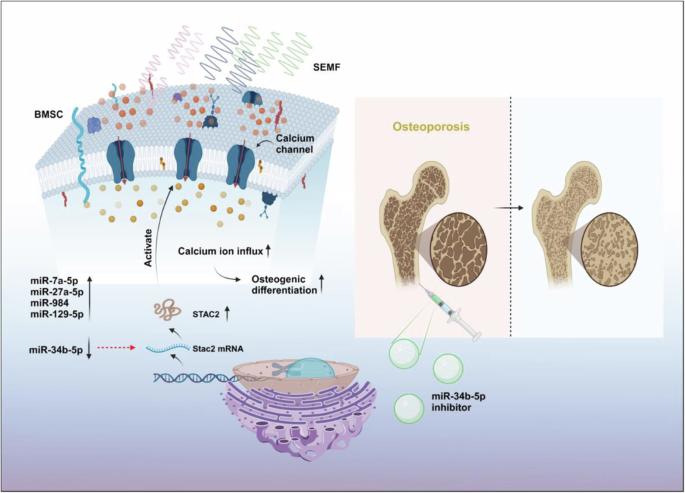
Low frequency sinusoidal electromagnetic fields promote the osteogenic differentiation of rat bone marrow mesenchymal stem cells by modulating miR-34b-5p/STAC2
Electromagnetic fields (EMFs) have emerged as an effective treatment for osteoporosis. However, the specific mechanism underlying their therapeutic efficacy remains controversial. Herein, we confirm the pro-osteogenic effects of 15 Hz and 0.4-1 mT low-frequency sinusoidal EMFs (SEMFs) on rat bone marrow mesenchymal stem cells (BMSCs). Subsequent miRNA sequencing reveal that miR-34b-5p is downregulated in both the 0.4 mT and 1 mT SEMFs-stimulated groups. To clarify the role of miR-34b-5p in osteogenesis, BMSCs are transfected separately with miR-34b-5p mimic and inhibitor. The results indicate that miR-34b-5p mimic transfection suppress osteogenic differentiation, whereas inhibition of miR-34b-5p promote osteogenic differentiation of BMSCs. In vivo assessments using microcomputed tomography, H&E staining, and Masson staining show that miR-34b-5p inhibitor injections alleviate bone mass loss and trabecular microstructure deterioration in ovariectomy (OVX) rats. Further validation demonstrates that miR-34b-5p exerts its effects by regulating STAC2 expression. Modulating the miR-34b-5p/STAC2 axis attenuate the pro-osteogenic effects of low-frequency SEMFs on BMSCs. These studies indicate that the pro-osteogenic effect of SEMFs is partly due to the regulation of the miR-34b-5p/STAC2 pathway, which provides a potential therapeutic candidate for osteoporosis. Low-frequency sinusoidal electromagnetic fields enhance osteogenesis in mice by regulating the miR34b-5p/STAC2 axis, providing insights into potential osteoporosis treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


