通过一次糖基化全合成结构复杂的脆弱拟杆菌多糖 B 的齐聚物六糖重复单元
IF 6.2
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
存在于常见肠道共生菌脆弱拟杆菌(Bacteroides fragilis)表面的聚合多糖(ZPSs)具有独特的免疫学特性,因为它们可以在没有蛋白质共轭的情况下直接与 T 细胞结合。因此,ZPSs 被认为是开发完全基于碳水化合物的疫苗的潜在抗原。在此,我们首次公开了脆弱拟杆菌的高支链磷酸化齐聚物荚膜多糖重复单元的全合成。这种含有六种不同单糖的六糖重复单元由三个 1,2-顺式糖苷键、D-QuipNAc-β-(1→4)-D-Gal 主题中一个具有挑战性的 1,2-反式键和一个 2-氨基乙基膦酸盐附属物组成。目标 ZPS 的合成采用了快速、高度立体选择性和收敛性(1 + 2 + 2 + 1)的一锅糖基化策略。其显著特点包括高效合成稀有的脱氧氨基糖 D- 和 L-喹诺酮胺,立体选择性地安装三个 1,2-顺式糖苷键,用立体拥挤、反应性差的 4-OH 半乳糖分子对 D-喹诺酮胺供体进行糖基化,以及后期的磷酸化。存在于常见肠道共生菌脆弱拟杆菌(Bacteroides fragilis)表面的聚合多糖被认为是开发完全基于碳水化合物的疫苗的潜在抗原。在此,作者报告了通过一锅糖基化策略全合成脆弱拟杆菌高支链磷酸化齐聚物荚膜六糖重复单元的情况。本文章由计算机程序翻译,如有差异,请以英文原文为准。
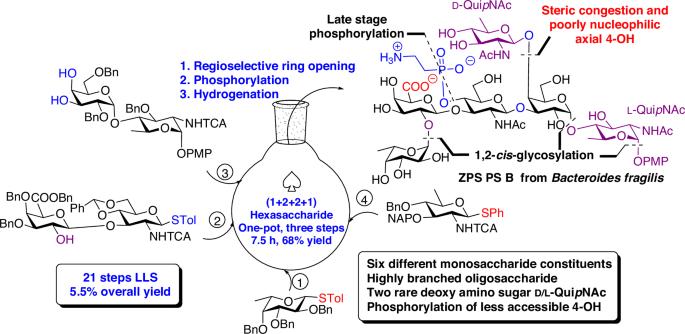
Total synthesis of a structurally complex zwitterionic hexasaccharide repeating unit of polysaccharide B from Bacteroides fragilis via one-pot glycosylation
Zwitterionic polysaccharides (ZPSs) present on the surface of a common gut commensal Bacteroides fragilis are endowed with unique immunological properties as they can directly bind to T-cells in the absence of protein conjugation. ZPSs are therefore considered to be potential antigens for the development of totally carbohydrate-based vaccines. Herein, we disclose the first total synthesis of a highly branched phosphorylated zwitterionic capsular polysaccharide repeating unit of Bacteroides fragilis. The hexasaccharide repeating unit bearing six different monosaccharides comprises three 1,2-cis-glycosidic linkages, a challenging 1,2-trans linkage in D-QuipNAc-β-(1→4)-D-Gal motif, and a 2-aminoethyl phosphonate appendage. The synthesis of target ZPS was accomplished utilizing an expeditious, highly stereoselective and convergent (1 + 2 + 2 + 1) one-pot glycosylation strategy. The striking features include efficient synthesis of rare deoxy amino sugars D- and L-quinovosamine, stereoselective installation of three 1,2-cis glycosidic linkages, glycosylation of D-quinovosamine donor with a sterically crowded, poorly reactive 4-OH galactose moiety, as well as late stage phosphorylation. Zwitterionic polysaccharides present on the surface of a common gut commensal Bacteroides fragilis are considered to be potential antigens for the development of totally carbohydrate-based vaccines. Here, the authors report the total synthesis of a highly branched phosphorylated zwitterionic capsular hexasaccharide repeating unit of Bacteroides fragilis via a one-pot glycosylation strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


