通过乙醇二聚反应利用二氧化碳生产正丁醇的前景
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
正丁醇等高级醇类可用作能源载体或化学品,因此是二氧化碳利用的理想产品。然而,从二氧化碳或通过合成气直接生成正丁醇却因产量低而受到阻碍。在此,我们设计了一种新颖的三步法,通过合成气和乙醇从二氧化碳生产正丁醇。我们还详细讨论了乙醇-丁醇-水混合物在较宽组成范围内的设计策略。工艺性能调查显示,合成气转化为乙醇对正丁醇的总年化成本(TAC)和二氧化碳利用效率(CUE)影响不大,但随着乙醇转化为正丁醇的转化率提高,TAC 和 CUE 稳步上升,分别达到 1739 美元/吨和 47%。为了获得正的 CUE,正丁醇的转化率至少应达到 20%,因此建议在正丁醇反应器周围采用旁路策略,以便从 40% 的转化率中获益。如果两个反应器的转化率都不高 (30-40%),二氧化碳成本应降至约 600-700 美元/吨,这样拟议的二氧化碳利用正丁醇工艺才可行。此外,一旦格尔伯特转化率达到 30-40%,进一步的催化开发应侧重于提高选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
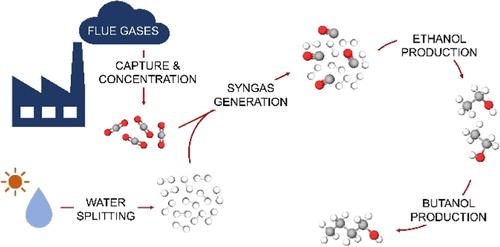
Prospects of n-Butanol Production from Carbon Dioxide via Ethanol Dimerization
Higher alcohols, such as n-butanol, are promising products of CO2 utilization due to their potential uses as energy carriers or chemicals. However, their direct formation from CO2 or via syngas is hindered by low yields. Here, we designed a novel 3-step process to produce n-butanol from CO2 via syngas and ethanol. Design strategies for an ethanol–butanol–water mixture over a wide composition range have also been discussed in detail. Process performance investigation revealed that the conversion of syngas to ethanol had little effect on the total annualized cost (TAC) of n-butanol and CO2 utilization efficiency (CUE), but TAC decreased and CUE increased steadily with the increasing conversion of ethanol to n-butanol to $1739/t and 47%, respectively. Conversion to n-butanol should be at least 20% for a positive CUE and a bypass strategy around the n-butanol reactor was proposed to benefit from >40% conversion. At modest conversions in both reactors (30–40%), the H2 cost should fall to about $600–700/t for the proposed n-butanol process from CO2 utilization to be viable. Moreover, once 30–40% Guerbet conversion is achieved, further catalysis development should focus on improving selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


