用于治疗伤口生物膜感染的工程噬菌体-聚合物纳米组件
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
溶菌噬菌体(噬菌体)的抗菌功效和特异性使其成为治疗耐多药细菌感染的有前途的疗法。然而,噬菌体穿透生物膜保护基质的能力有限,这可能会限制它们对生物膜感染的疗效。在这里,研究人员利用工程聚合物生成了非共价噬菌体-聚合物纳米组装体(PPNs),这种组装体能穿透细菌生物膜并杀死常驻细菌。噬菌体 K 对多种金黄色葡萄球菌(包括耐甲氧西林金黄色葡萄球菌 (MRSA))具有活性,它与阳离子聚氧乙烯聚合物组装成 PPNs。与游离噬菌体相比,PPNs 保留了噬菌体的感染性,同时显示出更强的生物膜穿透力和杀伤力。在体外,PPNs 对 MRSA 生物膜的杀灭率达到 3-log10(99.9%)。然后将 PPN 加入 Poloxamer 407(P407)水凝胶中,并应用于体内伤口生物膜,显示出可控的持续释放效果。融入水凝胶的 PPN 在小鼠 MRSA 伤口生物膜模型中效果显著,细菌量减少了 1.5-log10,而 P407 水凝胶中的噬菌体 K 只减少了 0.5 log。总之,这项工作展示了用阳离子聚合物设计的噬菌体 K 在治疗伤口生物膜感染方面的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
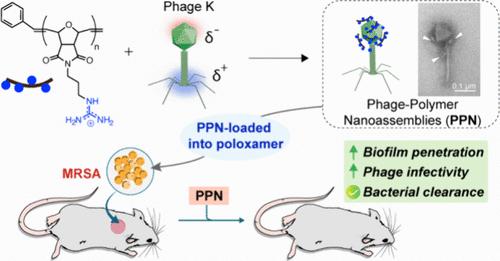
Engineered Bacteriophage-Polymer Nanoassemblies for Treatment of Wound Biofilm Infections
The antibacterial efficacy and specificity of lytic bacteriophages (phages) make them promising therapeutics for treatment of multidrug-resistant bacterial infections. Restricted penetration of phages through the protective matrix of biofilms, however, may limit their efficacy against biofilm infections. Here, engineered polymers were used to generate noncovalent phage-polymer nanoassemblies (PPNs) that penetrate bacterial biofilms and kill resident bacteria. Phage K, active against multiple strains of Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), was assembled with cationic poly(oxanorbornene) polymers into PPNs. The PPNs retained phage infectivity, while demonstrating enhanced biofilm penetration and killing relative to free phages. PPNs achieved 3-log10 bacterial reduction (∼99.9%) against MRSA biofilms in vitro. PPNs were then incorporated into Poloxamer 407 (P407) hydrogels and applied onto in vivo wound biofilms, demonstrating controlled and sustained release. Hydrogel-incorporated PPNs were effective in a murine MRSA wound biofilm model, showing a 1.5-log10 reduction in bacterial load compared to a 0.5 log reduction with phage K in P407 hydrogel. Overall, this work showcases the therapeutic potential of phage K engineered with cationic polymers for treating wound biofilm infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


