人类 48S 启动复合体控制翻译的结构基础
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
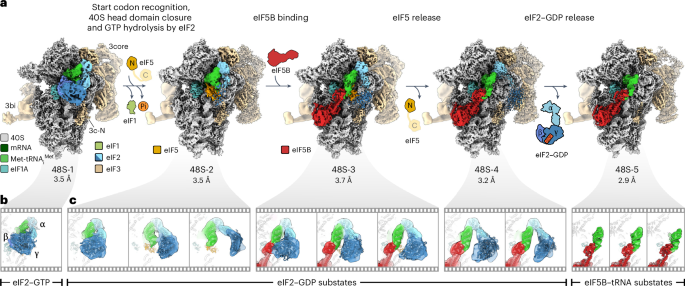
真核生物 mRNA 翻译开放阅读框(ORF)的选择依赖于扫描 48S 起始复合体重塑为延伸就绪的 80S 核糖体。我们利用低温电子显微镜观察了人类协调 48S 重塑的关键承诺步骤。mRNA Kozak 序列促进了 mRNA 在 48S 开放状态下的扫描,并通过组织真核启动因子(eIF)和核糖体蛋白的接触以及重构 mRNA 结构,稳定了 48S 封闭状态。GTPase 触发的 48S 结合 eIF2 的大规模波动促进了 eIF5B 的招募、启动子 tRNA 从 eIF2 转移到 eIF5B 以及 eIF5 和 eIF2 的释放。48S 结合的多亚基 eIF3 复合物通过将 eIF 交换与 eIF3c N 端结构域从亚基间界面逐渐移位结合起来,从而控制核糖体亚基的连接。这些发现揭示了人类细胞中ORF选择的结构机制,并解释了eIF3如何在80S核糖体中发挥作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Structural basis for translational control by the human 48S initiation complex
The selection of an open reading frame (ORF) for translation of eukaryotic mRNA relies on remodeling of the scanning 48S initiation complex into an elongation-ready 80S ribosome. Using cryo-electron microscopy, we visualize the key commitment steps orchestrating 48S remodeling in humans. The mRNA Kozak sequence facilitates mRNA scanning in the 48S open state and stabilizes the 48S closed state by organizing the contacts of eukaryotic initiation factors (eIFs) and ribosomal proteins and by reconfiguring mRNA structure. GTPase-triggered large-scale fluctuations of 48S-bound eIF2 facilitate eIF5B recruitment, transfer of initiator tRNA from eIF2 to eIF5B and the release of eIF5 and eIF2. The 48S-bound multisubunit eIF3 complex controls ribosomal subunit joining by coupling eIF exchange to gradual displacement of the eIF3c N-terminal domain from the intersubunit interface. These findings reveal the structural mechanism of ORF selection in human cells and explain how eIF3 could function in the context of the 80S ribosome. Cryo-electron microscopy reveals the mechanism of human translation initiation from codon scanning to subunit joining. The structures show the roles of the Kozak sequence, GTP hydrolysis by eukaryotic initiation factor 2 (eIF2) and eIF5B in 48S remodeling, as well as that of eIF3 in the control of 60S docking.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


