对 PD-1 表达进行表观遗传学调整可改善衰竭 T 细胞的功能和病毒控制能力
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
PD-1 是 CD8+ T 细胞活化的关键负调控因子,在癌症和慢性病毒感染中,衰竭的 T 细胞高度表达 PD-1。虽然 PD-1 阻断可改善病毒和肿瘤控制,但生理性 PD-1 表达可防止免疫病理并改善记忆形成。目前还不太清楚衰竭时 PD-1 高表达的驱动机制,这可能是区分其有益和有害影响的关键。在这里,我们利用一种删除衰竭特异性 PD-1 增强子的小鼠模型,对 PD-1 的表观遗传调控进行了功能性研究。与野生型和 Pdcd1 基因敲除细胞相比,增强子的缺失能完全改变慢性感染中 CD8+ T 细胞中 PD-1 的表达,创造出一个中间表达的 "甜蜜点",使 T 细胞的功能得到优化。这样就能更好地控制慢性感染,而不会产生额外的免疫病理反应。这些结果共同证明,通过表观遗传编辑调整 PD-1 可以减少 CD8+ T 细胞功能障碍,同时避免过多的免疫病理反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
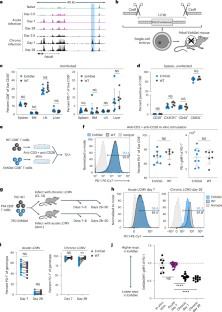
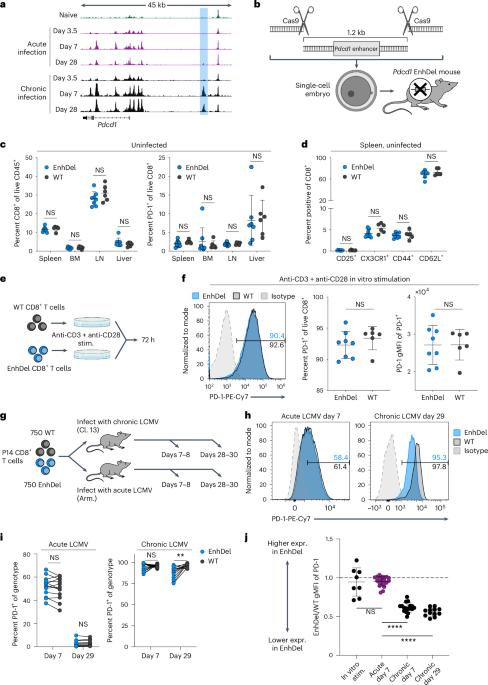
Epigenetic tuning of PD-1 expression improves exhausted T cell function and viral control
PD-1 is a key negative regulator of CD8+ T cell activation and is highly expressed by exhausted T cells in cancer and chronic viral infection. Although PD-1 blockade can improve viral and tumor control, physiological PD-1 expression prevents immunopathology and improves memory formation. The mechanisms driving high PD-1 expression in exhaustion are not well understood and could be critical to disentangling its beneficial and detrimental effects. Here, we functionally interrogated the epigenetic regulation of PD-1 using a mouse model with deletion of an exhaustion-specific PD-1 enhancer. Enhancer deletion exclusively alters PD-1 expression in CD8+ T cells in chronic infection, creating a ‘sweet spot’ of intermediate expression where T cell function is optimized compared to wild-type and Pdcd1-knockout cells. This permits improved control of chronic infection without additional immunopathology. Together, these results demonstrate that tuning PD-1 via epigenetic editing can reduce CD8+ T cell dysfunction while avoiding excess immunopathology. PD-1 is a critical modulator of CD8+ T cell activation and exhaustion. Sen and colleagues show that a cell-state-specific enhancer tunes PD-1 expression exclusively in exhaustion and that deletion of this enhancer improves CD8+ T cell function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


