一种二铁酶催化色氨酸与吲哚腈的氧化重排反应
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
腈在自然界中并不常见,通常是通过氨基酸底物的氧化脱羧作用或羧酸的衍生作用从肟中合成的。在这里,我们报告了以蓝藻腈合成酶 AetD 为特征的第三种腈类生物合成策略。在杀鹰神经毒素(aetokthonotoxin)的生物合成过程中,AetD 将 5,7-二溴-l-色氨酸的 2-氨基丙酸酯部分转化为腈类。通过综合运用结构、生物化学和生物物理技术,我们将 AetD 定性为一种非血红素二铁酶,属于新兴的血红素氧化酶样二金属氧化酶超家族。AetD 的高分辨率晶体结构以及催化相关产物的鉴定,从机理上揭示了 AetD 如何实现这种独特的转化,我们认为这种转化是通过氮丙啶中间体进行的。我们的工作为腈类的生物生成提供了一个独特的模板,并描绘了一种底物结合和金属因子组装机制,这种机制可能是其他血红素氧化酶类二元氧化酶所共有的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
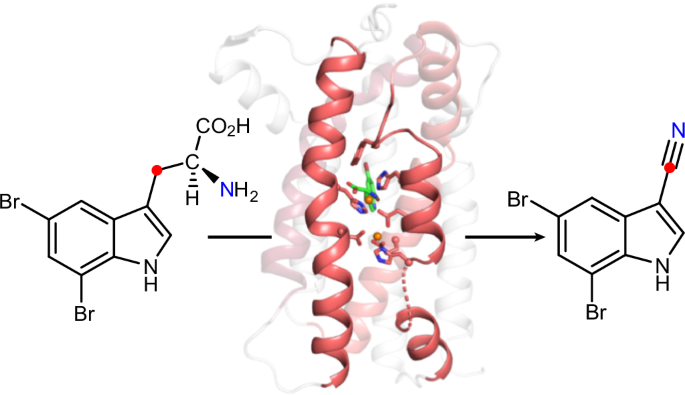
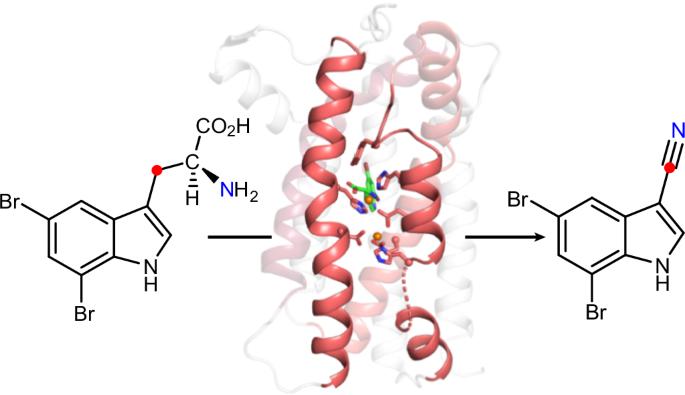
A single diiron enzyme catalyses the oxidative rearrangement of tryptophan to indole nitrile
Nitriles are uncommon in nature and are typically constructed from oximes through the oxidative decarboxylation of amino acid substrates or from the derivatization of carboxylic acids. Here we report a third nitrile biosynthesis strategy featuring the cyanobacterial nitrile synthase AetD. During the biosynthesis of the eagle-killing neurotoxin, aetokthonotoxin, AetD transforms the 2-aminopropionate portion of 5,7-dibromo-l-tryptophan to a nitrile. Employing a combination of structural, biochemical and biophysical techniques, we characterized AetD as a non-haem diiron enzyme that belongs to the emerging haem-oxygenase-like dimetal oxidase superfamily. High-resolution crystal structures of AetD together with the identification of catalytically relevant products provide mechanistic insights into how AetD affords this unique transformation, which we propose proceeds via an aziridine intermediate. Our work presents a unique template for nitrile biogenesis and portrays a substrate binding and metallocofactor assembly mechanism that may be shared among other haem-oxygenase-like dimetal oxidase enzymes. Nitrile-containing molecules and their biosynthetic enzymes are uncommon in nature. Now, a nitrile-forming diiron enzyme involved in the biosynthesis of aetokthonotoxin—the ‘eagle-killing’ neurotoxin—has been characterized using biochemical, structural and biophysical methods. High-resolution protein crystal structures together with the identification of catalytically relevant tryptophan-based products provide mechanistic insights into this unusual nitrile-forming reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


