从废旧锂离子电池中直接再生掺氟碳包覆的磷酸铁锂正极材料†。
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
随着磷酸铁锂电池(LFP)在电动汽车和能源储存领域的普及,人们开始关注其应用后的处置和回收问题。传统的回收方法存在经济和环境限制。直接回收是最有前途的方法。然而,不可逆的结构降解和不可避免的杂质阻碍了直接回收的实际应用。在此,我们提出了一种可持续的直接回收策略,即用甲醇-柠檬酸分离废电极碎片,然后通过残留的聚偏二氟乙烯(PVDF)修复分离出的 LFP。甲醇-柠檬酸溶剂可在室温下将电极废料完全分离为无损伤的废 LFP 和无腐蚀性的铝箔。通过固相烧结法,当废旧 LFP 材料中的 PVDF 含量为 5 wt%时,结晶度和微观结构得到很好的再生,并在再生的 LFP 颗粒上涂覆了掺氟碳三维导电网络结构。在再生 LFP 中保持稳定的导电炭黑可再次用于电池。再生的 LFP 正极材料显示出良好的放电能力(141.5 mA h g-1),100 次循环后,在 1C 下的保持率为 99.6%。我们的工作为回收废旧 LFP 提供了一种环保且具有成本效益的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
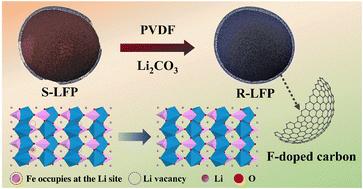
Direct regeneration of fluorine-doped carbon-coated LiFePO4 cathode materials from spent lithium-ion batteries†
The popularity of LiFePO4 (LFP) batteries in electric vehicles and energy storage has raised concerns about their disposal and recycling after application. Traditional recycling methods have economic and environmental limitations. Direct recycling is the most promising method. However, irreversible structural degradation and unavoidable impurities hinder the practical application of direct recycling. Here, a sustainable strategy, the methanol–citric acid separation of spent electrode scraps followed by the repair of the separated LFP through the residual polyvinylidene fluoride (PVDF), is proposed for direct recycling. The methanol–citric acid solvent can completely separate the electrode scraps into damage-free spent LFP and non-corrosive Al foil at room temperature. Through the solid-phase sintering method, as the PVDF content is 5 wt% in the spent LFP materials, the crystallinity and microstructure regenerate well, and a fluorine-doped carbon three-dimensional conductive network structure is coated on regenerated LFP particles. The conductive carbon black, which still remains stable in the regenerated LFP, is used again in the battery. The regenerated LFP cathode materials exhibit a good discharge capacity of 141.5 mA h g−1 and a retention rate of 99.6% at 1C after 100 cycles. Our work provides an environmentally friendly and cost-efficient strategy for the recovery of spent LFP.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


