解密可持续芬顿氧化作用的新型烟酸锰(II)/过氧单硫酸盐体系:烟酸锰(IV)过氧化单硫酸盐复合物的优势
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
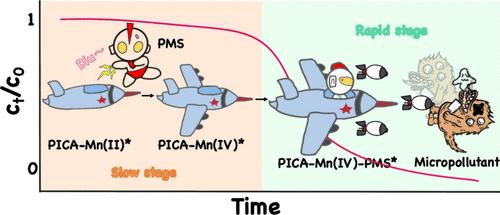
本文结合锰(II)、过氧化单硫酸盐(PMS)和可生物降解的吡啶甲酸(PICA),开发了一种高效、可持续的水处理系统。微污染物的消除过程分为两个阶段:最初的缓慢降解阶段(0-10 分钟)和随后的快速降解阶段(10-20 分钟)。多种证据表明,PICA-Mn(IV)复合物(PICA-Mn(IV)*)的产生起到了导电桥梁的作用,促进了 PMS 和微污染物之间的电子转移。量子化学计算显示,PMS 很容易将 PICA-Mn(II)* 氧化成 PICA-Mn(IV)*。然后,这种中间体与 PMS 复合,生成 PICA-Mn(IV)-PMS* ,从而拉长了 PMS 的 O-O 键,提高了其氧化能力。PICA-Mn(IV)-PMS* 介导的典型微污染物的主要转化机制包括氧化、开环、键裂和环氧化反应。毒性评估结果表明,大多数产物的毒性低于母体化合物。此外,Mn(II)/PICA/PMS 系统在实际水环境中表现出对水基质的适应性和高效性。值得注意的是,与传统的活性氧相比,PICA-Mn(IV)* 表现出更高的稳定性和更长的使用寿命,可以重复使用。总之,本研究开发了一种创新、可持续和选择性的氧化系统,即 Mn(II)/PICA/PMS,用于快速净化水,突出了原位生成的 Mn(IV)的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Deciphering the Novel Picolinate-Mn(II)/peroxymonosulfate System for Sustainable Fenton-like Oxidation: Dominance of the Picolinate-Mn(IV)-peroxymonosulfate Complex
A highly efficient and sustainable water treatment system was developed herein by combining Mn(II), peroxymonosulfate (PMS), and biodegradable picolinic acid (PICA). The micropollutant elimination process underwent two phases: an initial slow degradation phase (0–10 min) followed by a rapid phase (10–20 min). Multiple evidence demonstrated that a PICA-Mn(IV) complex (PICA-Mn(IV)*) was generated, acting as a conductive bridge facilitating the electron transfer between PMS and micropollutants. Quantum chemical calculations revealed that PMS readily oxidized the PICA-Mn(II)* to PICA-Mn(IV)*. This intermediate then complexed with PMS to produce PICA-Mn(IV)-PMS*, elongating the O–O bond of PMS and increasing its oxidation capacity. The primary transformation mechanisms of typical micropollutants mediated by PICA-Mn(IV)-PMS* include oxidation, ring-opening, bond cleavage, and epoxidation reactions. The toxicity assessment results showed that most products were less toxic than the parent compounds. Moreover, the Mn(II)/PICA/PMS system showed resilience to water matrices and high efficiency in real water environments. Notably, PICA-Mn(IV)* exhibited greater stability and a longer lifespan than traditional reactive oxygen species, enabling repeated utilization. Overall, this study developed an innovative, sustainable, and selective oxidation system, i.e., Mn(II)/PICA/PMS, for rapid water decontamination, highlighting the critical role of in situ generated Mn(IV).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


