大肠杆菌中 L-亮氨酸的多种效应导致在缺乏未磷酸化 PtsN 的情况下对 L-亮氨酸敏感的生长
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在大肠杆菌 K-12 中,未磷酸化 PtsN(unphospho-PtsN)的缺失被认为会导致对 L-亮氨酸敏感的生长表型(LeuS),其原因是 K+摄取过度活化介导的 ilvBN 操作子表达受损,该操作子编码对 L-缬氨酸(Val)敏感的乙酰羟基酸合成酶 I(AHAS I)的亚基,使残余的 AHAS 活性易受 Leu 和 K+的抑制。这导致了 AHAS 的不足和对 L-异亮氨酸(Ile)的需求。在此,我们为 ∆ptsN 突变体的 LeuS 提供了另一种机制。利用 LeuS 抑制剂进行的遗传学和生理学研究表明,由于缺乏非磷酸-PtsN 和存在 Leu,再加上 Leu 介导的 AHAS III 表达抑制,共同导致 ilvBN 操作子表达受损,从而导致 AHAS 不足,使残余的 AHAS 活性易受外源 Leu 可能产生的慢性 Val 压力的影响。超活化的 K+ 摄取和升高的 α-酮丁酸水平介导了 ilvBN 表达的升高并缓解了 LeuS。作为 ilvBN 表达的正向调节因子,unphospho-PtsN 的需要可能会缓冲 Ile 生物合成,防止 Leu 介导的 AHAS 不足,并保护 AHAS I 的功能免受 Leu 产生的慢性内源性 Val 的影响,在某些损害 AHAS 功能的环境中可能会实现这一点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
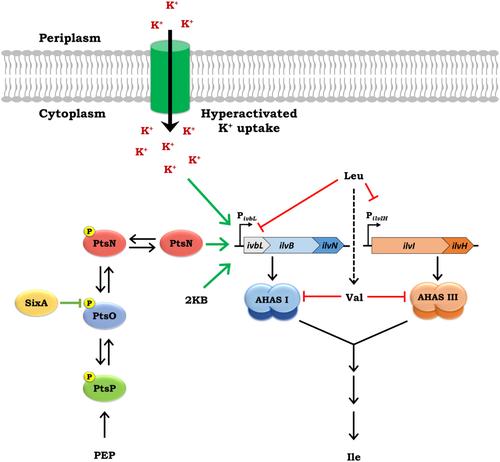
Multiple Effects of L-Leucine in Escherichia coli Lead to L-Leucine-Sensitive Growth in the Absence of Unphosphorylated PtsN
In E. coli K-12, the absence of unphosphorylated PtsN (unphospho-PtsN) has been proposed to cause an L-leucine-sensitive growth phenotype (LeuS) by hyperactivated K+ uptake mediated impairment of the expression of the ilvBN operon, encoding subunits of the L-valine (Val)-sensitive acetohydroxyacid synthase I (AHAS I) that renders residual AHAS activity susceptible to inhibition by Leu and K+. This leads to AHAS insufficiency and a requirement for L-isoleucine (Ile). Herein, we provide an alternate mechanism for the LeuS of the ∆ptsN mutant. Genetic and physiological studies with suppressors of the LeuS indicate that impaired expression of the ilvBN operon jointly caused by the absence of unphospho-PtsN and the presence of Leu coupled to Leu-mediated repression of expression of AHAS III leads to AHAS insufficiency rendering residual AHAS activity susceptible to chronic Val stress that may be generated by exogenous Leu. Hyperactivated K+ uptake and an elevated α-ketobutyrate level mediate elevation of ilvBN expression and alleviate the LeuS. The requirement of unphospho-PtsN as a positive regulator of ilvBN expression may buffer Ile biosynthesis against Leu-mediated AHAS insufficiency and protect AHAS I function from chronic endogenous Val generated by Leu and could be realized in certain environments that impair AHAS function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


