阴离子衍生接触离子配对作为电解质设计的统一原则
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
新型电化学技术的应用要求系统能够在要求越来越高的条件下运行,而储能应用方面的进展则为重新设计电解质以实现极端电位下的性能提供了诱人的机会。这些创新的一个共同点是在电解质中形成大量接触离子对(CIPs),而与具体的阳离子化学或溶剂系统无关。本综述中总结的例子表明,可能存在一套通用的电解质设计规则,有目的地选择阴离子化学性质可以产生可调节控制反应热力学、动力学和相间化学性质的 CIP 结构。利用实验和计算相结合的方法,并在机器学习和人工智能的辅助下,可以确定高性能阴离子衍生 CIP 结构的相关描述符,从而更迅速地调查现有的巨大组合空间,并为脱碳电化学过程大规模生产新一代电解质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
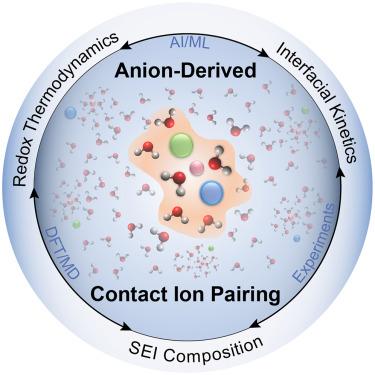
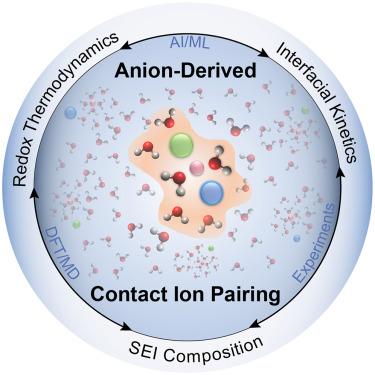
Anion-derived contact ion pairing as a unifying principle for electrolyte design
Enabling new electrochemical technologies requires systems that can operate under ever-more demanding conditions, and progress in energy storage applications reveals tantalizing opportunities to reimagine electrolyte design for performance at extreme potentials. A common thread among these innovations is the formation of significant populations of contact ion pairs (CIPs) in the electrolyte, regardless of the specific cation chemistry or solvent system. The examples summarized in this review suggest that a set of general electrolyte design rules likely exists, where the purposeful selection of anion chemistry can yield CIP structures with tunable control over reaction thermodynamics, kinetics, and interphase chemistry. Identifying the relevant descriptors for high-performance, anion-derived CIP structures can be achieved utilizing a combined experimental and computational approach, aided by machine learning and artificial intelligence, to more rapidly survey the vast combinatorial space available and to enable a new generation of electrolytes for decarbonized electrochemical processes at scale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


