通过微管聚合动态量化细胞器染色剂的细胞光毒性
IF 8.5
4区 医学
Q1 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
摘要
通过量化活细胞中染料和药物的光毒性,生物学家可以更好地了解细胞在成像过程中对外源刺激的反应。这种能力还有助于设计光毒性更低的荧光标签和药效更好的药物。评估细胞光毒性的传统方法依赖于对单个或不同细胞群的后期测量。在这里,我们开发了一种定量方法,利用细胞内微管聚合作为快速灵敏的标记来量化早期光毒性。利用这种方法,我们评估了细胞器染料在不同激发波长照射下诱导的光敏作用。值得注意的是,针对线粒体、细胞核和内质网的荧光标记表现出不同程度的光毒性。此外,利用实时精密光控技术,我们还能评估光和染料对特定细胞器的协同效应。在缺氧条件下进行的研究表明,Mito-Tracker Red CMXRos 的光毒性增强与活性氧的生成无关,而是与低氧条件下的不同有害途径有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
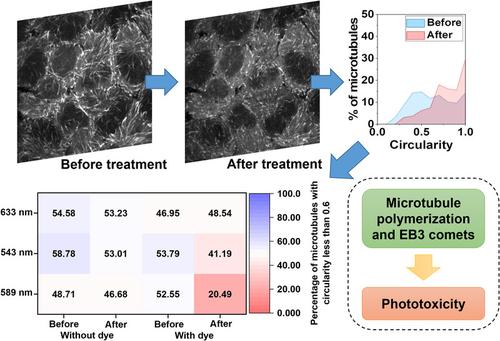
Quantification of cellular phototoxicity of organelle stains by the dynamics of microtubule polymerization
Being able to quantify the phototoxicity of dyes and drugs in live cells allows biologists to better understand cell responses to exogenous stimuli during imaging. This capability further helps to design fluorescent labels with lower phototoxicity and drugs with better efficacy. Conventional ways to evaluate cellular phototoxicity rely on late-stage measurements of individual or different populations of cells. Here, we developed a quantitative method using intracellular microtubule polymerization as a rapid and sensitive marker to quantify early-stage phototoxicity. Implementing this method, we assessed the photosensitization induced by organelle dyes illuminated with different excitation wavelengths. Notably, fluorescent markers targeting mitochondria, nuclei, and endoplasmic reticulum exhibited diverse levels of phototoxicity. Furthermore, leveraging a real-time precision opto-control technology allowed us to evaluate the synergistic effect of light and dyes on specific organelles. Studies in hypoxia revealed enhanced phototoxicity of Mito-Tracker Red CMXRos that is not correlated with the generation of reactive oxygen species but a different deleterious pathway in low oxygen conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

VIEW
Multiple-
CiteScore
12.60
自引率
2.30%
发文量
0
审稿时长
10 weeks
期刊介绍:
View publishes scientific articles studying novel crucial contributions in the areas of Biomaterials and General Chemistry. View features original academic papers which go through peer review by experts in the given subject area.View encourages submissions from the research community where the priority will be on the originality and the practical impact of the reported research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


