氧化还原中性镍催化的内炔烃选择性氢炔化反应及其在抗癌剂发现中的应用†。
IF 5.5
1区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
综合摘要本文通过氰基定向基团策略,在镍催化下实现了前所未有的非对称内部炔烃的区域选择性氢炔化反应,并具有立体受阻的选择性。值得注意的是,所得到的 1,3-炔产物可有效地用于合成新型含氮三环化合物,从而提供了具有抗肿瘤细胞增殖活性的潜在候选化合物 8a(IC50 = 2.6-6.1 μmol/L)。因此,这项工作不仅改进了过渡金属催化的内炔烃氢炔化策略,还展示了 1,3-炔烃在构建复杂生物活性化学空间方面的多功能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
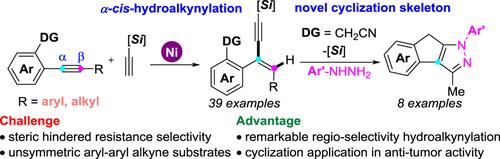
Redox-Neutral Nickel-Catalyzed Selective Hydroalkynylation of Internal Alkyne and Its Application in Anticancer Agent Discovery†
Herein, an unprecedented nickel-catalyzed regioselective hydroalkynylation of unsymmetrical internal alkynes was realized with steric hindered resistance selectivity via the cyano-directing group strategy. Significantly, the resulting 1,3-enyne products could be effectively employed in the synthesis of novel nitrogen-containing tricyclics compounds, that provided the potential candidate compound 8a (IC50 = 2.6—6.1 μmol/L) for the anti-tumor cell proliferation activity. Therefore, this work not only improves the transition-metal- catalyzed hydroalkynylation strategy of internal alkynes, but also exhibits versatility of 1,3-enynes in the construction of the complex bioactive chemical space.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemistry
化学-化学综合
CiteScore
8.80
自引率
14.80%
发文量
422
审稿时长
1.7 months
期刊介绍:
The Chinese Journal of Chemistry is an international forum for peer-reviewed original research results in all fields of chemistry. Founded in 1983 under the name Acta Chimica Sinica English Edition and renamed in 1990 as Chinese Journal of Chemistry, the journal publishes a stimulating mixture of Accounts, Full Papers, Notes and Communications in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


